01 March 2022: Clinical Research
Body Composition Changes and Related Factors in Patients with Ulcerative Colitis: A Retrospective Single-Center Study in China
Rongyu Wang1ABCDEFG, Xueli Ding1A, Zibin Tian1A, Xue JingDOI: 10.12659/MSM.933942
Med Sci Monit 2022; 28:e933942
Abstract
BACKGROUND: This study retrospectively explored body composition changes and related factors in patients with ulcerative colitis (UC).
MATERIAL AND METHODS: Patients with UC and healthy individuals who served as the healthy control at the Affiliated Hospital of Qingdao University September 2017 to August 2018 were retrospectively analyzed. Clinical data and laboratory examination indexes were collected. The skeletal muscle area (SMA) of the third lumbar vertebra cross-section, the subcutaneous fat area (SFA), and the visceral fat area (VFA) at the umbilical level were measured by computed tomography (CT), and the skeletal muscle index (SMI) was calculated to evaluate the loss of muscle mass.
RESULTS: Data from a total of 80 patients (median age, 49.49 years; 44 [55%] men) with active UC in the UC group and 80 healthy people age- and sex-matched in the healthy control group were collected. The incidence of low SMI and malnutrition was remarkably higher in the UC group than in the healthy control group (P<0.05). Low SMI was observed in 62.5% of UC patients who had a normal body mass index. Based on classification by the Truelove and Witts’ criteria, the prevalence of malnutrition in severe UC patients was remarkably higher than that in mild and moderate UC patients (P<0.05). Based on the disease extent, the prevalence of low SMI in E3 type UC was dramatically higher than that in E2 type (P=0.028).
CONCLUSIONS: Loss of muscle mass was related to disease extent in patients with UC. Loss of muscle mass is more likely to be associated with malnutrition.
Keywords: Body Composition, Inflammatory Bowel Diseases, malnutrition, China, Colitis, Ulcerative, Female, Humans, Incidence, Male, Tomography, X-Ray Computed
Background
Patients with inflammatory bowel disease (IBD), including ulcerative colitis (UC) and Crohn’s disease (CD), have altered body composition, such as diminished muscle mass, protein depletion, and higher abdominal adiposity [1]. These changes are due to a combination of inadequate intake, poor absorption, loss of nutrients, and increased demand for nutrients. These changes in body composition can lead to malnutrition, low muscle mass, sarcopenia, and unfavorable prognosis [2–5].
The criterion standard for evaluating muscle mass is dual-energy X-ray absorptiometry (DXA), computed tomography (CT), or magnetic resonance imaging (MRI) examinations. CT images provide details on specific muscles, adipose tissues, and organs, which are not provided by DXA. CT scanning has great practical significance as CT images were included in the diagnosis and follow-up [8,9]. The skeletal muscle cross-sectional area (SMA) at the level of the third lumbar vertebra (L3) measured by CT is considered to be a surrogate indicator of total skeletal muscle volume [9]. Studies have found the third lumbar cross-section skeletal muscle area is significantly correlated with whole-body muscle [9].
Body composition differs among racial and ethnic groups. At present, there are few studies on UC body composition in China. The objective of this study was to explore changes in body composition in patients with active UC and to investigate related influencing factors, with the goal of improving early detection of risk factors and prompt clinical interventions to increase the quality of life of UC patients.
Material and Methods
PATIENT SELECTION:
This retrospective study included patients with UC and healthy controls admitted to the Affiliated Hospital of Qingdao University (Qingdao, Shandong Province, China) from September 2017 to August 2018. This study was approved by the Ethics Committee of the Affiliated Hospital of Qingdao University (approval no. QYFY WZLL25852).
The inclusion criteria for the UC group were: (i) met diagnostic criteria based on the 2017 ECCO guidelines: established by clinical, laboratory, imaging, and endoscopic parameters, including histopathology, and people with other infections were excluded [10]; (ii) age ≥18 years old and ≤70 years old; (iii) complete data, including general information, laboratory data, and abdominal CT. Exclusion criteria were: (i) unable to be weighed; (ii) had other infections; (iii) pregnant women; (iv) patients with malignant tumors and other chronic diseases such as heart failure, chronic liver disease, diabetes, uremia, hyperthyroidism, and obstructive pulmonary disease; (v) incomplete clinical data or abdominal CT. In addition, UC patients who have been repeatedly admitted to the hospital are only admitted for the first time.
The inclusion criteria of the healthy control group were: (i) healthy people who came to our hospital’s physical examination center for physical examination during the same period, (ii) no disease, (iii) age ≥18 years old and ≤70 years old, and matched with UC group by age (±2 years) and sex, (iv) complete data, including general data, laboratory data, and abdominal CT. Exclusion criteria were: (i) had UC or any other diseases and infections, (ii) pregnant women, (iii) incomplete clinical data or abdominal CT.
DATA COLLECTION:
The clinical data on UC patients and healthy controls were collected and analyzed.
GENERAL INFORMATION OF PATIENTS: General information collected included age, sex, Montreal classification of extent of UC (Table 1) [11], and modified Truelove and Witts’ disease severity classification of active UC (Table 2) [12].
ANTHROPOMETRIC MEASUREMENTS:
On the day of abdominal CT examination, we measured height, weight, and BMI. According to the 2015 edition of the ESPEN guidelines, BMI <18.5 kg/m2 was defined as malnutrition [13,14].
LABORATORY DATA:
Collected laboratory data included white blood cell count (WBC), neutrophil count (Neu), lymphocyte count (Lym), red blood cell count (RBC), hemoglobin (Hb), platelet count (PLT), C-reactive protein (CRP), total protein (TP), albumin (ALB), pro-albumin (pro-ALB), and calcium ion (Ca2+). The detection time of the above data was on the day of or the day before the abdominal CT examination.
BODY COMPOSITION DETERMINATION BY CT:
The body composition was quantified by CT. CT scans show that the density of different tissues is different. Based on different density values, the skeletal muscle area, visceral fat area, and subcutaneous fat area can be accurately measured at a specific level. Before measurement, it needs to be calibrated according to the density value of water and air (the density value of water is 0 HU, and the density value of air is −1000 HU).
For measurement of SMA, we positioned the CT density threshold of skeletal muscle as −29 HU (Hounsfield unit) ~ +150 HU and measured all skeletal muscle regions (SMA) of the L3 cross-section, including the psoas major, erector spinae, quadratus lumborum, transverse abdominis, external oblique, and internal oblique (Figure 1A).
To measure SFA, the CT density threshold of subcutaneous and muscle fat was positioned as −190~−30 HU, and SFA was measured in the umbilical level (Figure 1B).
VFA was measured by positioning the CT density threshold of visceral fat as: −150~−50 HU, and VFA was measured at the umbilical level, which mainly includes the greater omentum, mesenteric, and intestinal fat groups (Figure 1C).
We calculated the L3 skeletal muscle index (SMI) as the ratio of SMA to height squared, which was used to assess skeletal muscle volume. Regarding the diagnostic criteria for low muscle mass, there is still controversy, and we used the standards widely used in Europe and the United States, in which SMI <52.4 cm2/m2 (male) or 38.5 cm2/m2 (female) indicates low muscle mass (low SMI) [15,17].
STATISTICAL ANALYSIS:
SPSS 24.0 statistical software (IBM, USA) was used for statistical analysis. Continuous data were compared by
Results
DEMOGRAPHIC AND CLINICOPATHOLOGICAL CHARACTERISTICS BETWEEN THE UC GROUP AND HEALTHY CONTROL GROUP:
A total of 160 participants were included in this study, with 80 in the UC group and 80 in the healthy control group. The average age of patients in the UC group was 49.49±1.37 years old, with a male to female ratio of 1.2: 1. The average age of the healthy control group was 49.58±1.34 years old.
The prevalence of low SMI and malnutrition were 68.8% and 20%, respectively, in the UC group. The prevalence of low SMI and malnutrition were 27.5% and 1.3%, respectively, in the healthy control group. Compared with the healthy group, the prevalence of low SMI and malnutrition were remarkably increased (P<0.05), and SMA, SFA, and VFA were significantly reduced in the UC group (P<0.05). Chi-square test analysis showed that low SMI patients were more likely to have malnutrition (P=0.016). The prevalence of low SMI in active UC patients with a normal BMI was as high as 62.5% (Table 3).
BODY COMPOSITION CHANGES AND NUTRITIONAL STATION WERE ANALYZED BASED ON TRUELOVE AND WITTS’ CRITERIA:
According to Truelove and Witts’ criteria, UC patients were divided into mild (S1), moderate (S2), and severe (S3) categories (Table 4). The prevalence of malnutrition in patients with S3 was significantly higher than that in patients with S1 (36.84% vs 5.00%, P<0.05) or S2 (36.84% vs 4.55%, P<0.05), and the same was true for body weight, BMI, RBC, HB, HCT, TP, ALB, pro-ALB, and Ca2+ (P<0.05). SMA and SFA were significantly lower in patients with S3 than in those with S1. There was no significant statistical difference between S1 and S2 and there was no significant difference in VFA among these groups.
BODY COMPOSITION CHANGES AND NUTRITIONAL STATION WERE ANALYZED BASED ON MONTREAL CLASSIFICATION:
According to the Montreal classification of UC lesions, UC patients were categorized into E1, E2, and E3 types (Table 5). The prevalence of low SMI in patients with E3 type UC was significantly higher than that in E2 type patients (79.55% vs 54.17%, P=0.028). TP, ALB, pro-ALB, and Ca2+ were significantly lower in E3 type UC than in E1 type UC (P<0.05). There was no significant statistical difference between E1 and E2. The height, BMI, SMI, TP, and ALB of low SMI UC patients were significantly lower than those of normal SMI patients (P<0.05). There was no significant difference in the incidence of malnutrition, SMA, SFA, VFA, and SMI among the groups.
RELATED FACTORS WITH SMI:
SMI was positively correlated with weight (ρ=0.659), BMI (ρ=0.646), RBC (ρ=0.514), HB (ρ=0.546), HCT (ρ=0.557), TP (ρ=0.444), ALB (ρ=0.571), pro-ALB (ρ=0.518), and Ca2+ (ρ=0.350), (respectively, P<0.05) and was negatively correlated with CRP (ρ=−0.4278, P=0.012); and had no correlation with age (Figure 2).
Discussion
This study showed that low muscle mass as assessed by CT was associated with the disease’s extent and severity in patients with UC. The presence of chronic inflammation can change the homeostasis of muscles, bones, and fat, and participate in the development of abnormal body composition and malnutrition. Increased energy expenditure due to inflammation, impaired digestive, and absorption function, as well as protein leakage due to ulcerative lesions, can lead to decreased skeletal muscle and fat volumes. Modified body composition, such as reduced fat-free mass, has been reported in patients with IBD [18–20].
Valentini et al analyzed the nutritional status and body composition of 114 patients with IBD, finding that 74% of patients had normal BMI, but body composition analysis showed that the body cell mass was reduced [19]. Our study analysis indicated that compared with the healthy control group, the skeletal muscle mass, subcutaneous fat mass, and visceral fat mass were significantly reduced, and the prevalence of low SMI and malnutrition was conspicuously increased in the UC group. The prevalence of low SMI in UC patients with normal BMI was as high as 62.5%. Thus, low SMI patients are more likely to have malnutrition. The results of our study suggested that patients with UC had more frequent skeletal muscle mass loss, which was more sensitive than the changes in BMI. Clinicians should assess loss of skeletal muscle mass rather than BMI changes in patients with UC to identify and treat those patients with a high risk for malnutrition. Yuqi et al also found that body composition measurements can effectively assess nutritional status and can be used as a supplement to BMI [21].
The present study showed that compared with mild or moderate UC patients, the prevalence of malnutrition was conspicuously increased, while TP, ALB, and Ca2+ were remarkably lower in patients with severe UC. Furthermore, SMA, SFA, and SMI were obviously lower in patients with severe UC than in mild UC patients. The prevalence of low SMI in UC patients classified as E3 by the Montreal classification was significantly higher than that in the E2 type patients. TP, ALB, and Ca2+ were significantly lower in the E3 type patients than in the E1 type patients. The symptoms of diarrhea and nausea in UC patients with severe UC or lesions involving the entire colon can result in less active intake, more intestinal loss, and reduced exercise. Previous studies have shown that exercise can improve the quality of life and reduce the clinical disease activity index of IBD patients [22–24]. By appropriately reducing disease activity, skeletal muscle mass can be improved [25,26]. This suggests that body composition analysis should be performed to identify loss of skeletal muscle mass as early as possible for patients with a wide range of lesions and severe symptoms, such as lowered serum albumin and blood calcium. Clinicians should improve UC patients’ nutritional status and quality of life by appropriately controlling inflammation, correcting malnutrition, and increasing exercise.
The results of the current study showed that BMI, SMI, SFA, TP, and ALB of UC patients with low SMI were significantly lower than those in patients with normal SMI. Skeletal muscle volume was closely related to sex, body weight, hemoglobin, serum albumin, and C-reactive protein levels, which is consistent with previous reports [27–30]. These results indicated that the skeletal muscle volume and serum albumin levels could be significantly reduced during acute and extensive disease [31]. When UC patients experience weight loss, anemia, decreased serum albumin, and significantly increased C-reactive protein, they should be examined to rule out muscular dystrophy. Body composition analysis should be performed as soon as possible to assess whether the patient’s skeletal muscle and fat mass are reduced. A study by Cushing et al showed that sarcopenia, as determined on abdominal CT, was a novel predictor of the need for rescue therapy in hospitalized UC patients [32].
This study has several limitations. Firstly, the number of cases was relatively small. Secondly, this was a retrospective study, so we could not measure muscle performance by grip-strength tests or walking speed and could not assess the prevalence of sarcopenia. Finally, we did not evaluate the possible treatments these patients may receive, which might change their body composition measurements and laboratory test results. Future research is necessary to confirm our results.
Conclusions
Malnutrition and loss of skeletal muscle mass were associated with disease extent in patients with UC. The UC active disease period can cause skeletal muscle loss and fat consumption and increase the risk of malnutrition. Body composition analysis could provide early detection of skeletal muscle loss and malnutrition in UC patients, as well as guide prompt clinical nutrition intervention.
Figures
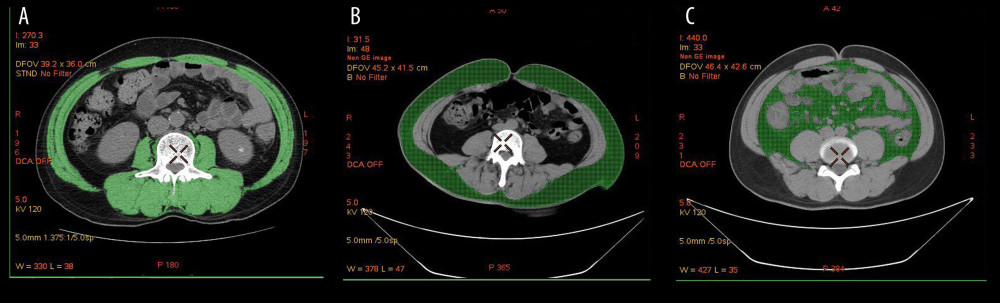 Figure 1. Body composition determination by CT. (A) The green part represents the skeletal muscle area (SMA) of the third lumbar vertebra cross-section. (B) The green part represents the subcutaneous fat area (SFA) at the umbilical level. (C) The green part represents the visceral fat area (VFA) at the umbilical level.
Figure 1. Body composition determination by CT. (A) The green part represents the skeletal muscle area (SMA) of the third lumbar vertebra cross-section. (B) The green part represents the subcutaneous fat area (SFA) at the umbilical level. (C) The green part represents the visceral fat area (VFA) at the umbilical level. 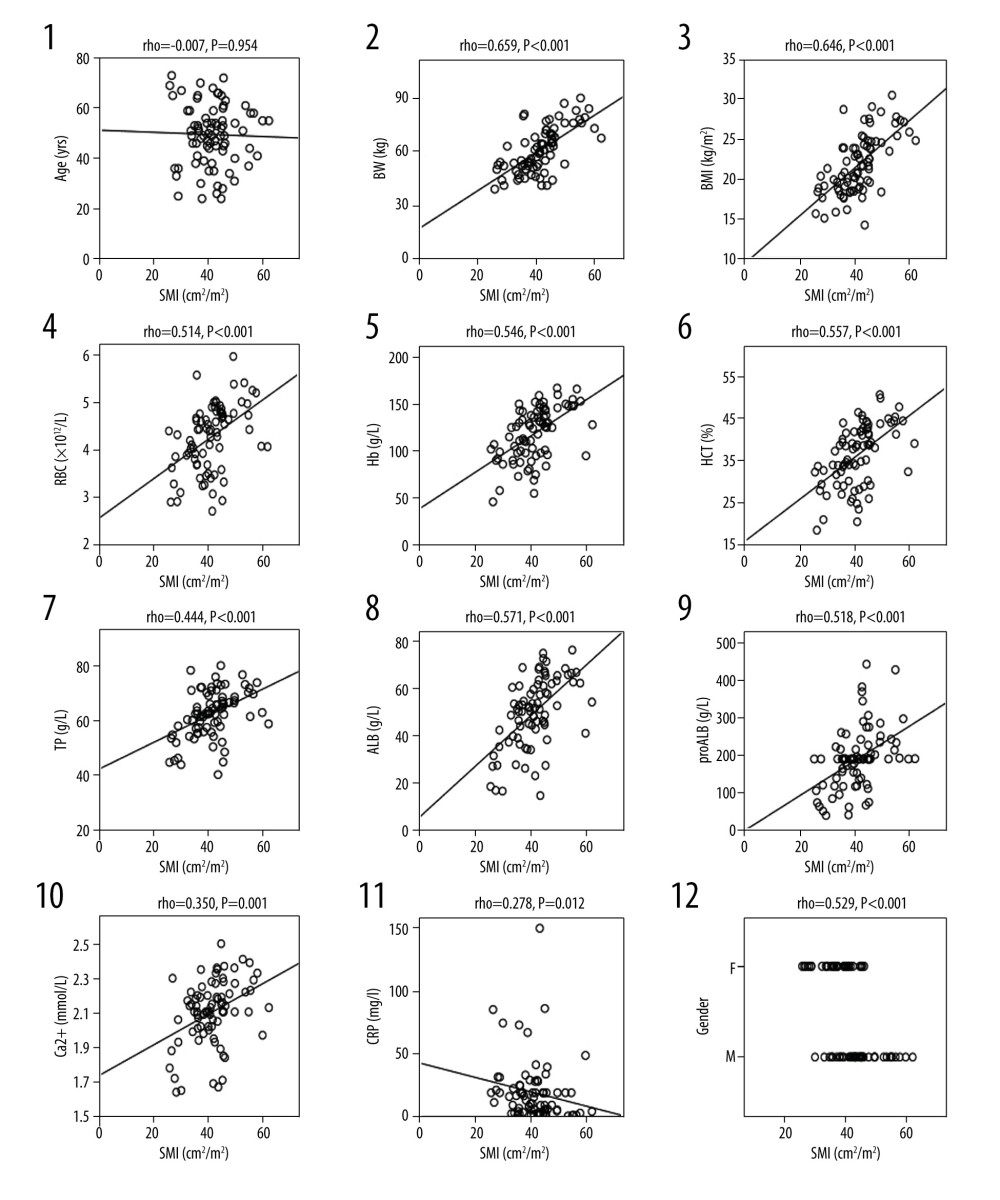 Figure 2. Correlation analysis between SMI and clinical variables. Clinical variables include: age (1), weight (2), BMI (3), red blood cell count (4), hemoglobin (5), hematocrit (6), total protein (7), serum white protein (8), serum prealbumin (9), Ca2+ (10), C-reactive protein (11), and sex (12). SMI – Skeletal Muscle Index; P<0.05 has statistical significance.
Figure 2. Correlation analysis between SMI and clinical variables. Clinical variables include: age (1), weight (2), BMI (3), red blood cell count (4), hemoglobin (5), hematocrit (6), total protein (7), serum white protein (8), serum prealbumin (9), Ca2+ (10), C-reactive protein (11), and sex (12). SMI – Skeletal Muscle Index; P<0.05 has statistical significance. Tables
Table 1. Montreal classification of extent of UC.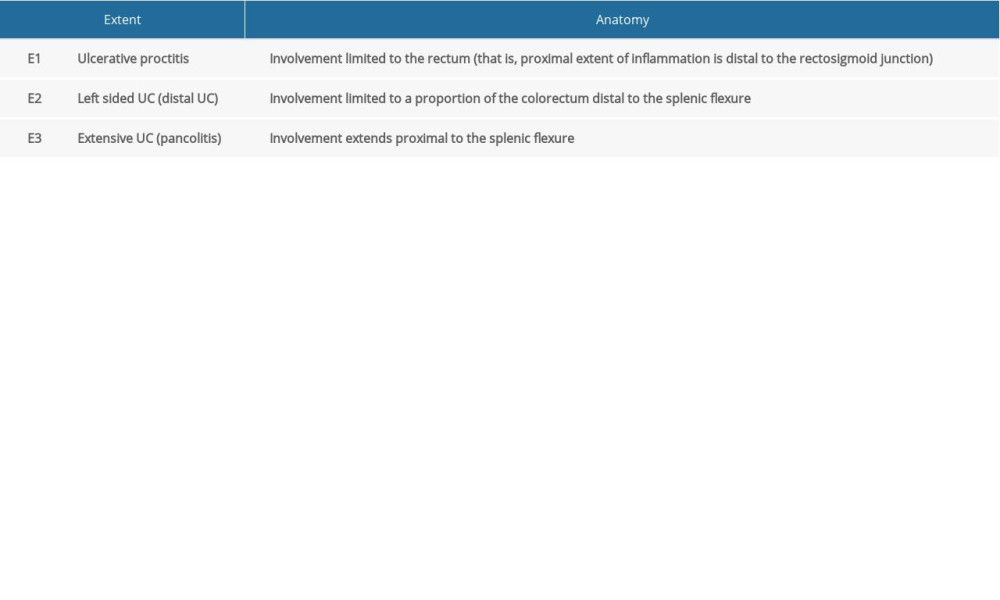 Table 2. Modified Truelove and Witts’ disease severity classification of UC.
Table 2. Modified Truelove and Witts’ disease severity classification of UC.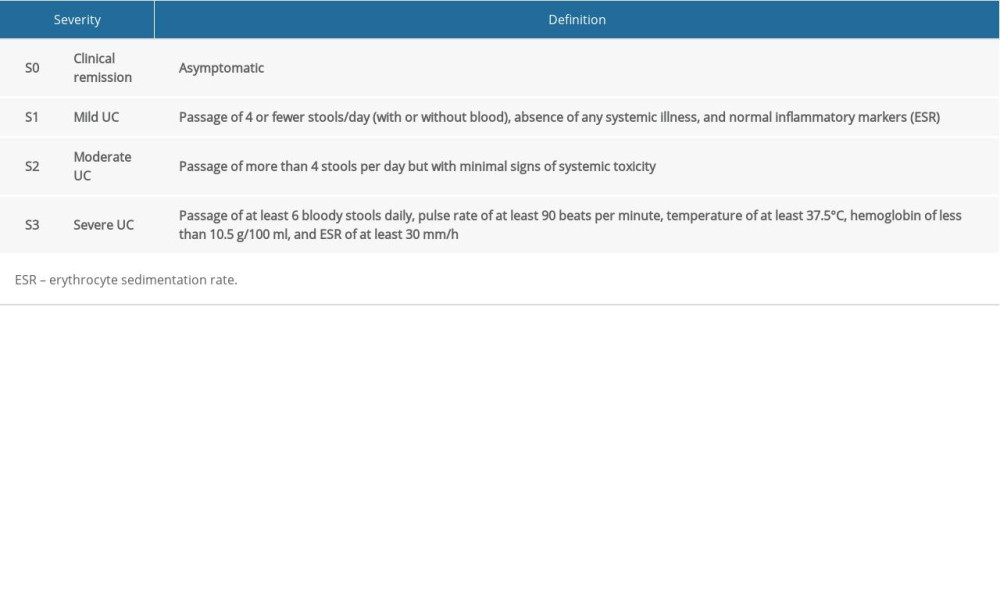 Table 3. Clinical and body composition parameters for UC patients and controls.
Table 3. Clinical and body composition parameters for UC patients and controls.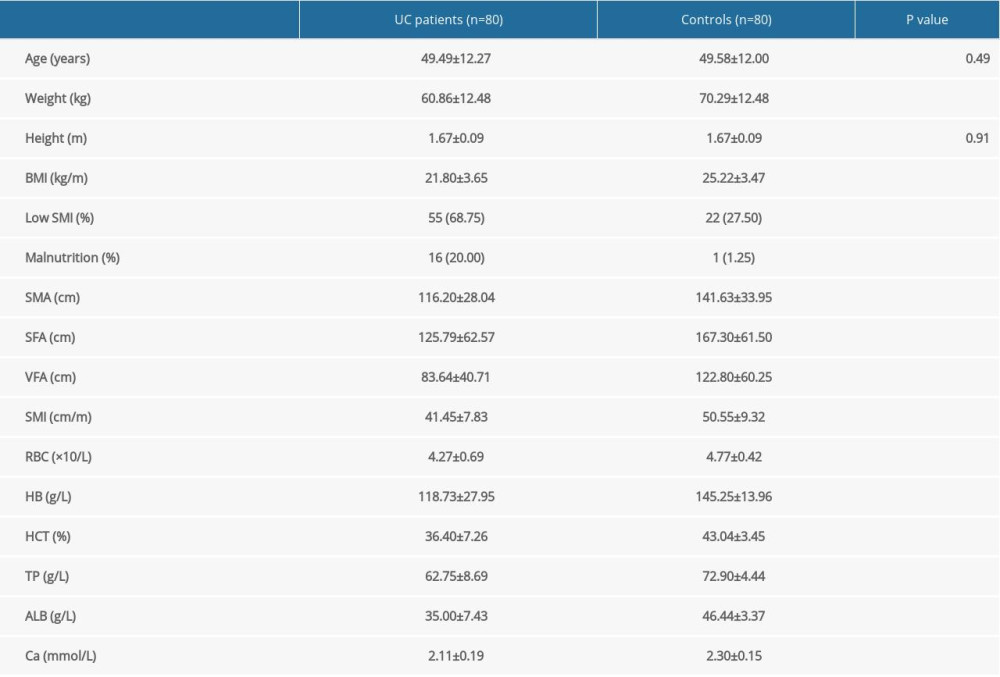 Table 4. Comparative analysis of UC patients grouped by disease activity.
Table 4. Comparative analysis of UC patients grouped by disease activity.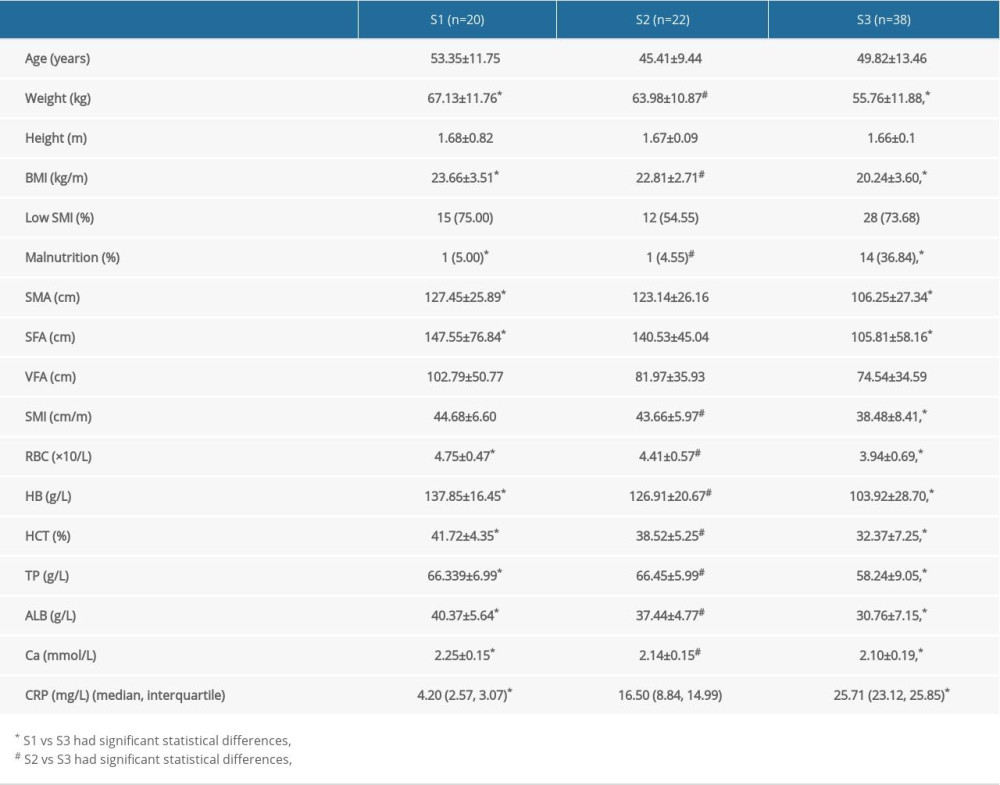 Table 5. Comparison and analysis of UC patients grouped by Montreal classification.
Table 5. Comparison and analysis of UC patients grouped by Montreal classification.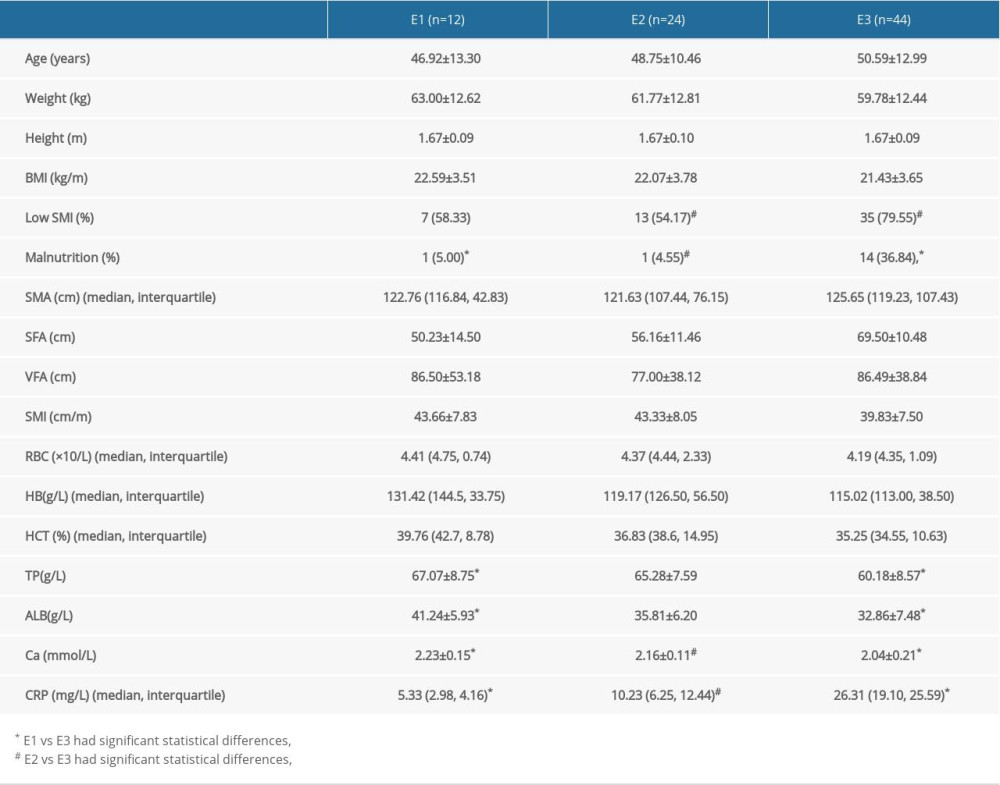
References
1. Kemp K, Griffiths J, Lovell K, Understanding the health and social care needs of people living with IBD: A meta-synthesis of the evidence: World J Gastroenterol, 2012; 18; 6240-49
2. Terzoudis S, Zavos C, Koutroubakis IE, The bone and fat connection in inflammatory bowel diseases: Inflamm Bowel Dis, 2014; 20; 2207-17
3. Costamagna D, Costelli P, Sampaolesi M, Penna F, Role of inflammation in muscle homeostasis and myogenesis: Mediators Inflamm, 2015; 2015; 805172
4. Goncalves P, Magro F, Martel F, Metabolic inflammation in inflammatory bowel disease: Crosstalk between adipose tissue and bowel: Inflamm Bowel Dis, 2015; 21; 453-67
5. Scaldaferri F, Pizzoferrato M, Lopetuso LR, Nutrition and IBD: Malnutrition and/or sarcopenia? A practical guide: Gastroenterol Res Pract, 2017; 2017; 8646495
6. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People: Age Ageing, 2010; 39; 412-23
7. Bryant RV, Ooi S, Schultz CG, Low muscle mass and sarcopenia: Common and predictive of osteopenia in inflammatory bowel disease: Aliment Pharmacol Ther, 2015; 41; 895-906
8. Benton CR, Wright DC, Bonen A, PGC-1alpha-mediated regulation of gene expression and metabolism: Implications for nutrition and exercise prescriptions: Appl Physiol Nutr Metab, 2008; 33; 843-62
9. Fearon K, Strasser F, Ankser SD, Definition and classification of cancer cachexia: An international consensus: Lancet Incol, 2011; 12; 489-95
10. Magro F, Gionchetti P, Eliakim R, Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: Definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders: J Crohns Colitis, 2017; 11; 649-70
11. Satsangi J, Silverberg MS, Vermeire S, Colombel JF, The Montreal classification of inflammatory bowel disease: Controversies, consensus, and implications: Gut, 2006; 55; 749-53
12. Truelove SC, Witts LJ, Cortisone in ulcerative colitis; Final report on a therapeutic trial: Br Med J, 1955; 2; 1041-48
13. Cederholm T, Bosaeus I, Barazzoni R, Diagnostic criteria for malnutrition – An ESPEN Consensus Statement: Clin Nutr, 2015; 34; 335-40
14. Mijac DD, Jankovic GL, Jorga J, Krstic MN, Nutritional status in patients with active inflammatory bowel disease: Prevalence of malnutrition and methods for routine nutritional assessment: Eur J Intern Med, 2010; 21; 315-19
15. Zhang T, Cao L, Cao T, Prevalence of sarcopenia and its impact on postoperative outcome in patients with Crohn’s disease undergoing bowel resection: J Parenter Enteral Nutr, 2017; 41; 592-600
16. Nishikawa H, Shiraki M, Hiramatsu A: Hepatol Res, 2016; 46; 951-63
17. Reid MB, Li YP, Tumor necrosis factor-alpha and muscle wasting: A cellular perspective: Respir Res, 2001; 2; 269-72
18. Bryant RV, Trott MJ, Bartholomeusz FD, Andrews JM, Systematic review: Body composition in adults with inflammatory bowel disease: Aliment Pharmacol Ther, 2013; 38; 213-25
19. Valentini L, Schaper L, Buning C, Malnutrition and impaired muscle strength in patients with Crohn’s disease and ulcerative colitis in remission: Nutrition, 2008; 24; 694-702
20. Csontos AA, Molnar A, Piri Z, Malnutrition risk questionnaire combined with body composition measurement in malnutrition screening in inflammatory bowel disease: Rev Esp Enferm Dig, 2017; 109; 26-32
21. Yuqi Q, Mi Z, Jun S, Zhihua R, Bioelectrical impedance analysis for body composition and nutritional status in hospitalized patients with inflammatory bowel disease: Chin J Gastroenterol, 2019; 24; 5
22. Loudon CP, Corroll V, Butcher J, The effects of physical exercise on patients with Crohn’s disease: Am J Gastroenterol, 1999; 94; 697-703
23. Ng V, Millard W, Lebrun C, Howard J, Low-intensity exercise improves quality of life in patients with Crohn’s disease: Clin J Sport Med, 2007; 17; 384-88
24. Cosnes J, Smoking, physical activity, nutrition and lifestyle: Environmental factors and their impact on IBD: Dig Dis, 2010; 28; 411-17
25. Zhang T, Ding C, Xie T, Skeletal muscle depletion correlates with disease activity in ulcerative colitis and is reversed after colectomy: Clin Nutr, 2017; 36; 1586-92
26. Bryant RV, Ooi S, Schultz CG, Low muscle mass and sarcopenia: Common and predictive of osteopenia in inflammatory bowel disease: Aliment Pharmacol Ther, 2015; 41; 895-906
27. Prado CM, Lieffers JR, McCargar LJ, Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study: Lancet Oncol, 2008; 9; 629-35
28. Montano-Loza AJ, Meza-Junco J, Prado CM, Muscle wasting is associated with mortality in patients with cirrhosis: Clin Gastroenterol Hepatol, 2012; 10; 166-73
29. Hanai T, Shiraki M, Nishimura K, Sarcopenia impairs prognosis of patients with liver cirrhosis: Nutrition, 2015; 31; 193-99
30. Zhuang CL, Huang DD, Pang WY, Sarcopenia is an independent predictor of severe postoperative complications and long-term survival after radical gastrectomy for gastric cancer: Analysis from a large-scale cohort: Medicine (Baltimore), 2016; 95; e3164
31. Carter MJ, Lobo AJ, Travis SP, Guidelines for the management of inflammatory bowel disease in adults: Gut, 2004; 53(Suppl 5); V1-16
32. Cushing KC, Kordbacheh H, Gee MS, Sarcopenia is a novel predictor of the need for rescue therapy in hospitalized ulcerative colitis patients: J Crohns Colitis, 2018; 12; 1036-41
Figures
 Figure 1. Body composition determination by CT. (A) The green part represents the skeletal muscle area (SMA) of the third lumbar vertebra cross-section. (B) The green part represents the subcutaneous fat area (SFA) at the umbilical level. (C) The green part represents the visceral fat area (VFA) at the umbilical level.
Figure 1. Body composition determination by CT. (A) The green part represents the skeletal muscle area (SMA) of the third lumbar vertebra cross-section. (B) The green part represents the subcutaneous fat area (SFA) at the umbilical level. (C) The green part represents the visceral fat area (VFA) at the umbilical level. Figure 2. Correlation analysis between SMI and clinical variables. Clinical variables include: age (1), weight (2), BMI (3), red blood cell count (4), hemoglobin (5), hematocrit (6), total protein (7), serum white protein (8), serum prealbumin (9), Ca2+ (10), C-reactive protein (11), and sex (12). SMI – Skeletal Muscle Index; P<0.05 has statistical significance.
Figure 2. Correlation analysis between SMI and clinical variables. Clinical variables include: age (1), weight (2), BMI (3), red blood cell count (4), hemoglobin (5), hematocrit (6), total protein (7), serum white protein (8), serum prealbumin (9), Ca2+ (10), C-reactive protein (11), and sex (12). SMI – Skeletal Muscle Index; P<0.05 has statistical significance. Tables
 Table 1. Montreal classification of extent of UC.
Table 1. Montreal classification of extent of UC. Table 2. Modified Truelove and Witts’ disease severity classification of UC.
Table 2. Modified Truelove and Witts’ disease severity classification of UC. Table 3. Clinical and body composition parameters for UC patients and controls.
Table 3. Clinical and body composition parameters for UC patients and controls. Table 4. Comparative analysis of UC patients grouped by disease activity.
Table 4. Comparative analysis of UC patients grouped by disease activity. Table 5. Comparison and analysis of UC patients grouped by Montreal classification.
Table 5. Comparison and analysis of UC patients grouped by Montreal classification. Table 1. Montreal classification of extent of UC.
Table 1. Montreal classification of extent of UC. Table 2. Modified Truelove and Witts’ disease severity classification of UC.
Table 2. Modified Truelove and Witts’ disease severity classification of UC. Table 3. Clinical and body composition parameters for UC patients and controls.
Table 3. Clinical and body composition parameters for UC patients and controls. Table 4. Comparative analysis of UC patients grouped by disease activity.
Table 4. Comparative analysis of UC patients grouped by disease activity. Table 5. Comparison and analysis of UC patients grouped by Montreal classification.
Table 5. Comparison and analysis of UC patients grouped by Montreal classification. In Press
05 Mar 2024 : Clinical Research
Role of Critical Shoulder Angle in Degenerative Type Rotator Cuff Tears: A Turkish Cohort StudyMed Sci Monit In Press; DOI: 10.12659/MSM.943703
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








