21 July 2022: Clinical Research
Serum T Cell Immunoglobulin Mucin 3 Predicts Worse Prognosis in Hepatocellular Carcinoma Patients Undergoing Transcatheter Arterial Chemoembolization
Jun Li1BCDE, Jing Wang1ABCEF*DOI: 10.12659/MSM.935326
Med Sci Monit 2022; 28:e935326
Abstract
BACKGROUND: Hepatocellular carcinoma (HCC) is currently a leading cause of cancer-related death, and its prognostic evaluation remains a challenge. We aimed to explore the predictive value of T cell immunoglobulin and mucin 3 (Tim-3) in HCC patients undergoing transcatheter arterial chemoembolization (TACE).
MATERIAL AND METHODS: For the present study, 167 HCC patients who had undergone TACE were retrospectively recruited and randomly stratified into 2 subgroups at a ratio of about 2: 1 (training: 109; validation: 58) using EXCEL software. Pre-TACE serum was collected from all of these patients, and in a second visit a month after the initial treatment, an additional serum sample was collected from 45 patients in the training set. Tim-3 concentrations were determined by enzyme-linked immunosorbent assay. Kaplan-Meier survival curves analysis, log-rank tests, and Cox proportional hazard regression model were applied to evaluate prognostic significance.
RESULTS: Patients whose cancer progressed after TACE had significantly higher serum Tim-3 in both the training and validation sets (both P<0.05). Kaplan-Meier analysis revealed that patients with elevated serum Tim-3 had significantly shorter time-to-progression and overall survival in both the training and validation sets (both P<0.05). Multivariate Cox regression analysis confirmed that elevated pre-TACE Tim-3 served as a novel, independent indicator for predicting poor prognosis in HCC [progression, hazard ratio (HR) 2.197; 95% confidential interval (CI) 1.408-3.373; P<0.001; death, HR 2.570; 95% CI 1.508-4.378; P=0.001], which was further verified in the validation set (progression P=0.005; death P=0.043). Interestingly, serum Tim-3 retained its prognostic significance in the a-fetoprotein-negative subgroup. Notably, patients with high Tim-3 levels after TACE encountered dismal outcomes.
CONCLUSIONS: Serum Tim-3 might be a satisfactory prognostic indicator for HCC patients undergoing TACE.
Keywords: HAVCR2 Protein, Human, Carcinoma, Hepatocellular, Prognosis, Chemoembolization, Therapeutic, Hepatitis A Virus Cellular Receptor 2, Humans, Immunoglobulins, Liver Neoplasms, Mucin-3, T-Lymphocytes, Treatment Outcome
Background
Hepatocellular carcinoma (HCC) is the fifth most common malignancy and the third leading cause of cancer-related death worldwide [1]. Surgical resection remains the major approach for treating HCC, but only early HCC patients are suitable for this treatment [2]. Unfortunately, although diagnostic strategy has been updated in recent years, only a minority of HCC patients benefit from early screening, and most HCC patients are diagnosed at an advanced stage. The prognosis of these unresectable HCC patients is relatively poor, evidenced by the median overall survival (OS), which ranges from 7 to 37 months [3]. Conventionally, transcatheter arterial chemoembolization (TACE), an intraarterial catheter-based chemotherapy that selectively delivers drugs to tumor lesions while embolizing the artery, is considered as among the most optimal therapeutic approaches for unresectable HCC patients [4]. However, the response after TACE varies greatly and OS also remains unsatisfactory for these patients, with the approximate 5-year survival rates of TACE ranging from 1–8% [5]. Considering that the treatment is individualized, to improve management efficiency, it is important to identify useful biomarkers for predicting response before applying TACE treatment. However, until now, no reliable serum biomarker has been identified to exert high efficiency in predicting the prognosis of HCC patients who received TACE.
Serum protein markers are considered as the most applicable targets for routine clinical assessments for cancer surveillance due to advantages such as non-invasiveness, lower serum requirement, cost efficiency, and high reproducibility [6]. Escaping immune surveillance is considered as the main driver for cancer progression [7], and secreted immune-related molecules that mediate interactions between the immune system and solid tumors have proven to be ideal circulating biomarkers for predicting prognosis and tumor progression in various types of cancer in HCC [8–10]. Recently, T cell immunoglobulin and mucin domain-3 (Tim-3) was identified as a key regulator involved in tumor immunological tolerance [11–13]. Functional investigations revealed that Tim-3 expression was closely associated with T cell dysfunction and exhaustion, while the blockade of interaction between Tim-3 and its ligand could effectively restore T cell functions, such as IFN-γ production [14]. Tim-3 is also expressed in a subpopulation of regulatory T cells which exert impressive immunosuppressive capacity in the tumor microenvironment, and this immunosuppression is closely correlated with poor prognosis [15]. Importantly, recent studies have unveiled that Tim-3 can enter peripheral circulation either by active secretion or passive shedding, and that the serum Tim-3 level can also exhibit prognostic value when considering solid tumors [16–18], suggesting that soluble Tim-3 might be a promising biomarker for evaluating prognosis in HCC. However, until now, there has been no evidence demonstrating the clinical relevance of serum Tim-3 in HCC patients receiving TACE.
In light of the above considerations, we speculated that serum Tim-3 might also be a useful biomarker with the potential to predict prognosis in HCC patients undergoing TACE, and we retrospectively recruited 167 HCC patients who had received TACE, and stratified these patients randomly into the 2 subgroups at an approximate ratio of 2: 1 (training set: 109; validation set: 58) to systematically evaluate the value of serum Tim-3.
Material and Methods
PATIENTS AND CLINICAL SAMPLES:
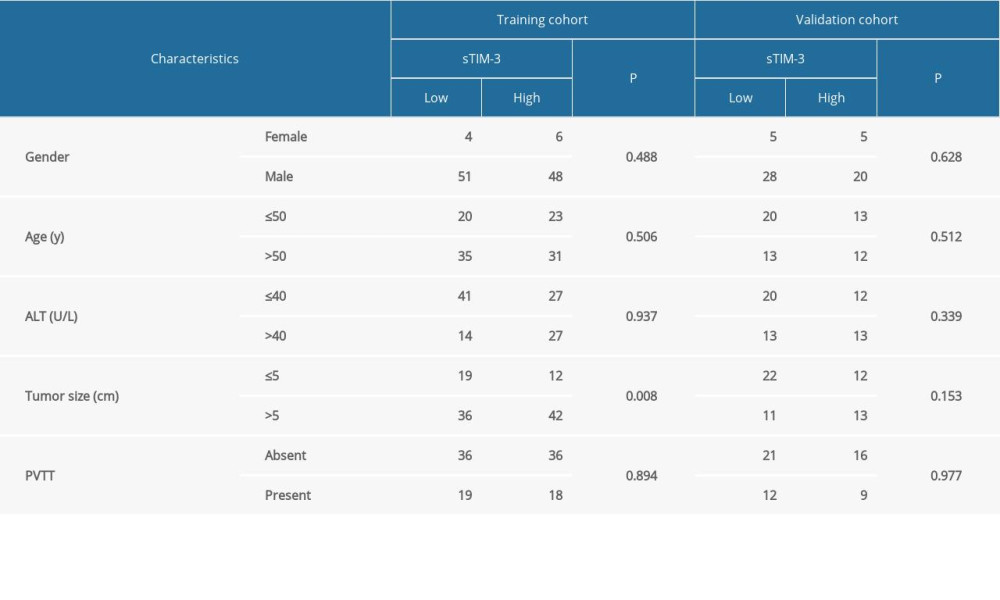
First, regarding the sample size of the present study, from April 2014 to December 2015, a total of 167 HCC patients underwent TACE. These patients were retrospectively recruited for our study and randomly stratified into 2 subgroups at an approximate ratio of 2: 1 (training set: 109; validation set: 58) using Microsoft EXCEL software. Briefly, all patients were randomly assigned a number as an individual barcode, then we sorted the patients ascendingly by the barcodes. The top 109 patients were stratified into the training set, while the rest of the patients were stratified into the validation set. Enrollment criteria were as follows: 1) definitive HCC diagnosis, 2) no prior treatment history, 3) received TACE treatments targeting intrahepatic lesions, and 4) availability of complete clinicopathologic and followup data. Exclusion criteria: failure to meet any of the above inclusion criteria. For HCC diagnosis, imaging scans, especially enhanced computed tomography (CT) or magnetic resonance imaging (MRI) scans, with typical characteristics of arterial phase enhancement and an alpha-fetoprotein (AFP) test, were taken into consideration according to the American Association for the Study of Liver Disease guidelines. Demographic and clinical data are listed in Table 1. In addition, another 20 healthy donors and 20 resectable HCC patients were also recruited as controls. The present study was performed in accordance with the 1975 Declaration of Helsinki and the Reporting Recommendations for Tumor Marker (REMARK) guidelines. Approval for the use of human subjects was obtained from the research ethics committee of People’s Hospital of Ningxia Hui Autonomous Region, and informed consent was obtained from each enrolled individual. Serum samples were collected 1–2 days before TACE, and centrifuged as soon as they were received at 2000×g for 10 min at 4°C. Afterwards, centrifuged samples were aliquoted and stored at −80°C until serum Tim-3 concentration determination.
FOLLOW-UP:
Post-treatment surveillance was conducted as previously described [19]. In brief, during the first half year, patients were followed up every month, then followup was conducted every 3 to 4 months. All patients received abdominal ultrasonography and chest X-ray at an interval of 1 to 6 months, depending on the time post-treatment. CT scans were conducted every 6 months. Bone scan or MRI scans were conducted if localized bone pain was found. Followup was ended in October 2019. Treatment efficacy was evaluated by modified Response Evaluation Criteria in Solid Tumors (mRECIST). Time to progression (TTP) was defined as the interval between the date of receiving TACE and the date of first reporting disease progression, or the date of the last followup visit if progression did not occur. OS was defined as the interval between the date of TACE and the reported date of death, or the date of last observation if patients did not suffer death.
SERUM TIM-3 CONCENTRATION MEASUREMENT:
Serum Tim-3 concentrations were determined by a sandwich enzyme-linked immunosorbent assay (ELISA) using the AbCam proprietary SimpleStep ELISA® technology kit (Human TIM3 ELISA Kit, AbCam, ab231932, Cambridge, USA), according to the manufacturer’s instructions. Generally, this was a one-step, single-wash, sandwich ELISA kit designed for the determination of serum or plasma Tim-3 concentration, which was suitable for our present study. First, serum samples were double diluted, and then 50 μL of samples or standards were added into the appropriate wells. Next, 50 μL of antibody cocktail, including capture and detector antibodies, were added into each well containing the samples or standards. The whole plate was than incubated at room temperature for 1 hour, followed by aspiration and washing 3 times with 350 μL 1× Wash Buffer provided in the detection kit. Afterwards, 100 μL of tetramethyl benzidine (TMB) Development Solution was added into each well and incubated in the well for 10 min at room temperature. Finally, 100 μL of Stop Solution was added into each well to stop the reaction and the optimal density of each well was measured by a spectrophotometer (Multiskan Go, Thermo Fisher, USA; purchased from Shanghai, China). Standard curves were established by conducting regression analysis based on all optical density values of the standards. Then, detailed concentrations of serum Tim-3 were calculated by plotting the optical density value of each sample on the standard curve and extrapolating to find the concentration. The operator who performed the experiments was blinded to the serum sample information.
STATISTICAL ANALYSIS:
Statistical analysis was conducted using SPSS 26.0 software (IBM, USA; downloaded from the library of Ningxia Medical University in China). Continuous variables were expressed as the mean±standard derivation. First, all grouped data underwent an F-test to determine whether the variances were homogeneous. If the variances were homogeneous, a
Results
SERUM TIM-3 WAS ELEVATED IN HCC PATIENTS UNDERGOING TACE AND SHOWED NO CORRELATION WITH SERUM AFP:
First, serum Tim-3 concentrations among healthy donors, resectable HCC patients, and HCC patients who underwent TACE were compared. Serum Tim-3 concentration in HCC patients was significantly higher than that in healthy donors (mean: 5128.8 for TACE: 3131 for resectable vs 850.9 ng/ml, both P<0.05). More importantly, HCC patients who underwent TACE showed an even higher serum Tim-3 level (significantly higher) than that of the resectable HCC patients (mean: 5128.8 vs 3131.3 ng/ml, P<0.05, Figure 1A), indicating that serum Tim-3 might be correlated with the severity of HCC progression. Moreover, the correlation between serum Tim-3 and AFP, a widely used serum biomarker for HCC diagnosis as well as prognosis evaluation, was assessed. Serum Tim-3 concentration showed no significant association with serum AFP, in both the training and validation sets (Figure 1B, 1C), suggesting that Tim-3 might serve as a surrogate biomarker that could supplement or complement AFP in the clinical practice of HCC management.
HIGHER SERUM TIM-3 CONCENTRATION CORRELATED WITH POOR PROGNOSIS:
We further evaluated whether serum Tim-3 was associated with worse prognosis in HCC patients who received TACE. We found that patients who suffered from progression after primary TACE treatment had significantly higher serum Tim-3 levels than patients without progression, in the training set (Figure 2A). When we stratified patients into 2 subgroups according to the median concentration of serum Tim-3; into the training set as serum Tim-3high (>4475.1 pg/ml) and serum Tim-3low (≤4475.1 pg/ml) subgroups, we found that serum Tim-3high patients had higher progression incidence than serum Tim-3low patients (98.15% vs 72.73%, Figure 2B). Similarly, patients who died had significantly higher serum Tim-3 levels than patients who lived (Figure 2C), and serum Tim-3high patients had higher death incidence than serum Tim-3low patients (79.63% vs 40.00%, Figure 2D).
In the validation set, patients who suffered progression or death also had a higher serum Tim-3 level, and serum Tim-3high patients showed a higher progression or death rate than serum Tim-3low patients, which resembled the results in the training set (Figure 2E–2H).
HIGHER SERUM TIM-3 CONCENTRATION INDICATED SHORTER TTP AND OS AFTER TACE:
As elevated serum Tim-3 correlated with higher progression and death rates, we further investigated the prognostic value of serum Tim-3 in HCC patients who underwent TACE, via Kaplan-Meier curve analysis. As expected, when stratified according to median value of serum Tim-3 concentration in the training set, we found patients with high serum Tim-3 had significantly shorter TTP in both the training (median TTP 7.0 vs 17.6 months, P<0.001, Figure 3A) and the validation (median TTP 13.3 vs 22.3 months, P=0.010, Figure 3B) sets. Moreover, patients with high serum Tim-3 also exhibited significantly shorter OS in both the training (median TTP 23.0 months vs unreached, P<0.001, Figure 3C) and the validation (median TTP 25.6 vs 29.5 months, P=0.014, Figure 3D) sets. The above data further indicated the clinical significance of serum Tim-3 in prognosis after TACE treatment.
PRE-TACE SERUM TIM-3 SERVED AS AN INDEPENDENT INDICTOR FOR TTP AND OS IN HCC:
Next, univariate Cox regression analysis was conducted. In the training set, serum Tim-3 (P<0.001), portal vein tumor thrombosis (PVTT) (P<0.001), and serum AFP level (P=0.002, Figure 4A) showed prognostic significance for tumor progression. Also, all 3 of these parameters showed prognostic significance for OS (serum Tim-3: P<0.001; PVTT: P<0.001; serum AFP: P=0.039; Figure 4B). In the validation set, however, we observed serum Tim-3 as the only parameter with prognostic significance for both TTP (P=0.013, Figure 4C) and OS (P=0.016, Figure 4D).
Of note, serum Tim-3 was confirmed as an independent indicator for both progression [hazard ratio (HR) 2.197; 95% confidential interval (CI) 1.408–3.373; P<0.001; Figure 4E] and death (HR 2.570; 95%; CI 1.508–4.378; P=0.001, Figure 4F) in the training set. Supporting these results, in the validation set, serum Tim-3 was also verified as an independent indicator for both progression (HR 2.510; 95%; CI 1.315–4.791; P=0.005, Figure 4G) and death (HR 2.007; 95% CI 1.023–3.939; P=0.043, Figure 4H). Together, the above results confirmed pretreatment serum Tim-3 as a novel, independent prognostic marker in patients receiving TACE.
PRE-TACE SERUM TIM-3 ALSO EXHIBITED PROGNOSTIC SIGNIFICANCE IN THE LOW-AFP SUBGROUP:
We further explored prognostic value of pretreatment serum Tim-3 in the low-AFP subgroup. The results indicated that patients with high serum Tim-3 had significantly shorter TTP in both the training (median TTP; 12.0 vs 19.0 months, P<0.001, Figure 5A) and validation (median TTP 14.2 vs 22.3 months; P=0.014, Figure 5B) sets. In addition, patients with high serum Tim-3 also exhibited significantly shorter OS, in both the training (median TTP 24.0 months vs unreached, P=0.002, Figure 5C) and validation (median TTP 26.1 vs 39.5 months, P=0.003, Figure 5D) sets. The above data demonstrated that serum Tim-3 level also exerts significant prognosis prediction value in the low-progression-risk subgroup.
PROGNOSTIC VALUE OF DYNAMIC ALTERATION OF SERUM TIM-3 IN HCC PATIENTS WHO RECEIVED TACE:
We further explored the dynamic changes in serum Tim-3 during the peri-TACE period (before and 1 month after TACE) in 45 patients in the training set. Unfortunately, we did not observe any significant decrease in serum Tim-3 when measured 1 month after TACE, according to paired t test (data not shown). Therefore, we assessed whether the dynamic changes in the positive states before and after TACE shed influence on prognosis evaluation. These 45 patients were further stratified into 4 subgroups based on peri-TACE serum Tim-3 levels: Group I, high for both pretreatment and post-treatment; Group II, pretreatment high and post-treatment low; Group III, pretreatment low and post-treatment high; and Group IV, remained low after TACE. Interestingly, we found that patients with persistent, high serum Tim-3 had significantly shorter TTP and lower OS than patients who showed a pattern of decreased serum Tim-3 (TTP: P=0.009, Figure 6A; OS: P=0.005, Figure 6B). Moreover, patients who showed an increased serum Tim-3 pattern had significantly shorter TTP and lower OS than patients with persistent low serum Tim-3 (TTP: P=0.012, Figure 6A; OS: P=0.034, Figure 6B). However, no statistical significance was observed between the other subgroups. Collectively, our data demonstrated that reduced serum Tim-3 level might be a promising indicator for better prognosis, whereas increased alteration pattern might reflect a worse prognosis.
Discussion
Predicting prognosis after TACE via baseline parameters has been a long-term problem that puzzles clinical decision-makers. Identification of useful baseline parameters for accurately predicting treatment response might facilitate personalized therapy for patients with advanced-stage HCC. In the present study, we report that pretreatment serum Tim-3, an easily tested target via ELISA assays, was significantly elevated in HCC patients who then encountered adverse outcomes such as rapid progression or shorter OS after TACE treatment. Importantly, our data demonstrated that pre-TACE serum Tim-3 served as a novel, independent indicator of poor prognosis in HCC patients undergoing TACE. Moreover, monitoring of dynamic changes in serum Tim-3 during the peri-TACE period might provide accurate information for predicting long-term prognosis. Among the many types of circulating parameters, non-protein circulating biomarkers have been identified – for example, cell-free DNAs or cell-free RNAs. However, circulating protein biomarkers remain the most applicable detection target for routine clinical use, because generally such detection methods are non-invasive, require a relatively low volume of specimen, are not dependent on an operator with high expertise, are cost-efficient, have high reproducibility, and have extremely simple sample-pretreatment protocol (for example, no need for extraction or prior purification procedures) [6]. Hence, application of serum Tim-3 detection might be a useful approach for improving HCC management in the future.
Tim-3, firstly identified in 2002, is a well-established immune checkpoint molecule that exerts strong inhibitory effects on anti-cancer immunity by repressing cytotoxic T cell proliferation, and reducing the production of effector cytokines of these T cells [20–22]. As a cell membrane surface molecule, Tim-3 is likely to fall off from the membrane surface due to cell migration or rapid metabolism, and then enter the circulation where it can be detected. As evidence for this, recently, it was also identified as a circulating biomarker involved in the progression of several diseases. For example, increased circulating Tim-3 has been reported to be correlated with the progression of sepsis [23]. Moreover, circulating Tim-3 concentration was also shown to be associated with severe graft-versus-host disease [24] and cutaneous systemic sclerosis [25]. Interestingly, elevated circulating Tim-3 concentration correlated significantly with non-relapse mortality and OS in patients who received allogeneic hematopoietic cell transplantation [26]. More importantly, in cancerous situations, circulating Tim-3 has been reported to be elevated in patients with colorectal cancer and osteosarcoma [27,28]. In HCC, increased serum Tim-3 level has been identified as a novel prognostic indicator for patients undergoing curative resection [18]. In middle- or advanced-stage HCC patients who received TACE, serum Tim-3 level was elevated when HCC stage escalated [17]. However, the prognostic value of serum Tim-3 remained elusive in HCC patients receiving TACE. Here, we provided evidence that detection of pre-treatment serum Tim-3 might provide useful information for predicting poor prognosis after TACE. Our data was in accordance with previous studies, and further extended the clinical relevance of serum Tim-3, demonstrating that determination of serum Tim-3 might screen out who might benefit from TACE, and those who have elevated pre-treatment serum Tim-3 might benefit from more frequent surveillance and followup after TACE.
In clinical practice, AFP remains the most popular serum biomarker for HCC [29]. Unfortunately, about 30 to 40% of HCC patients showed low serum AFP (<400 ng/ml) when diagnosed, indicating a strong need for novel circulating biomarkers for these AFP-low patients [30–32]. Therefore, the predictive performance of serum Tim-3 in the low-AFP subgroup is of great potential benefit. Our data showed that detection of serum Tim-3 could further stratify AFP-low patients into 2 subgroups, termed serum Tim-3 low or serum Tim-3 high, and more importantly, individuals in the serum Tim-3 low subgroup also had significantly shorter TTP and OS than patients in the serum Tim-3 high subgroup. Our findings demonstrated that serum Tim-3 is a promising prognostic biomarker for HCC, especially for patients with low serum AFP. Detection of pre-TACE serum Tim-3 can help the clinicians to identify patients at high risk of progression who need more careful surveillance or additional therapy.
There were several limitations of our present study. First, due to lack of sufficient samples collected from healthy donors, we could barely construct the normal range and optimal cutoff value for serum Tim-3 to discriminate HCC patients from healthy individuals, a project being concurrently undertaken in our center. Second, we also could not determine the precise origin of serum Tim-3 in HCC patients. Since Tim-3 could be expressed on the surface of several types of cells, the origin of serum Tim-3 could be confirmed by conducting immunohistochemistry staining in HCC tissues. However, we failed to obtain resected HCC tissues in the present study. Nonetheless, we could still observe that patients with decreased serum Tim-3 levels had better prognoses, suggesting a close association between serum Tim-3 and tumor burden. We will further evaluate this association in our future work. Finally, most HCC patients in China have a background of hepatitis B virus infection, which differs greatly from the patient population in the United States, Europe, or Japan, according to previous studies [6]. Hence, the prognostic value of serum Tim-3 needs further verification in patients with different etiologies and clinical histories.
Conclusions
To the best of our knowledge, the present study is the first investigation to demonstrate the prognostic predictive value of serum Tim-3 in HCC patients who have received TACE treatment. Further investigation into the function of serum Tim-3 in regulating systemic immune cells, especially with regard to CD8+ T cell exhaustion, might provide a novel insight into the mechanism of HCC immunotolerance and immune evasion by HCC cells. Collectively, determination of serum Tim-3 concentration during the TACE treatment period might be useful for improving prognosis of HCC patients.
Figures
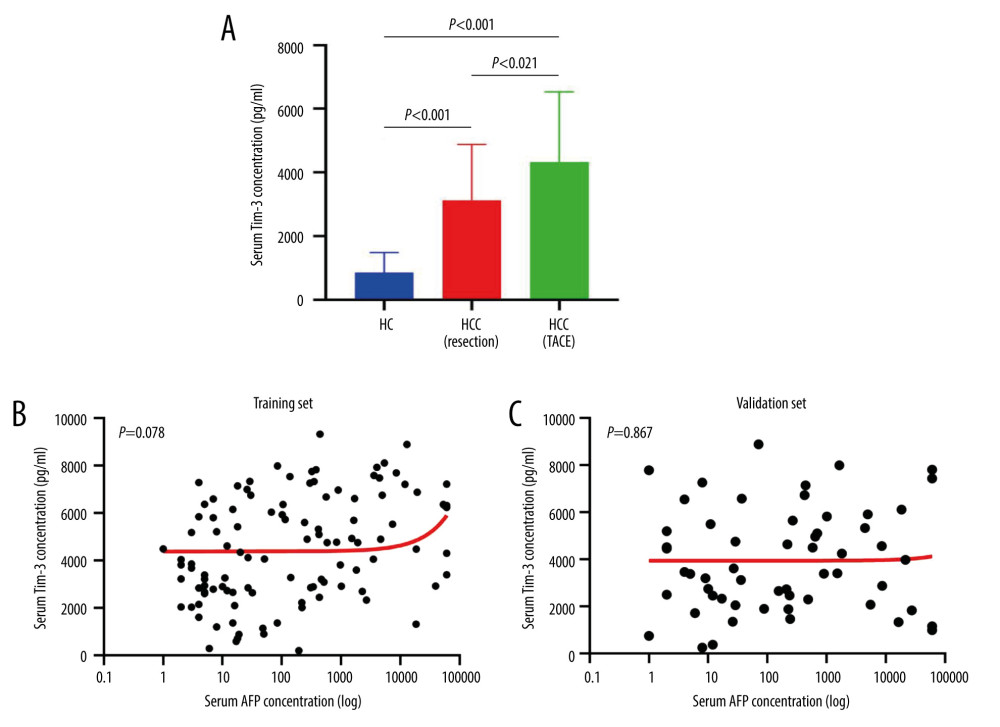 Figure 1. Serum Tim-3 distribution and correlation with serum AFP level. (A) Distribution of serum Tim-3 in healthy controls, resectable HCC patients, and unresectable HCC patients receiving TACE treatment. (B) Correlation between serum Tim-3 and AFP in the training set. (C) Correlation between serum Tim-3 and AFP in the validation set. AFP – alpha-fetoprotein; HC – healthy controls; HCC – hepatocellular carcinoma; TACE – transcatheter arterial chemoembolization; Tim-3 – T cell immunoglobulin and mucin domain 3.
Figure 1. Serum Tim-3 distribution and correlation with serum AFP level. (A) Distribution of serum Tim-3 in healthy controls, resectable HCC patients, and unresectable HCC patients receiving TACE treatment. (B) Correlation between serum Tim-3 and AFP in the training set. (C) Correlation between serum Tim-3 and AFP in the validation set. AFP – alpha-fetoprotein; HC – healthy controls; HCC – hepatocellular carcinoma; TACE – transcatheter arterial chemoembolization; Tim-3 – T cell immunoglobulin and mucin domain 3. 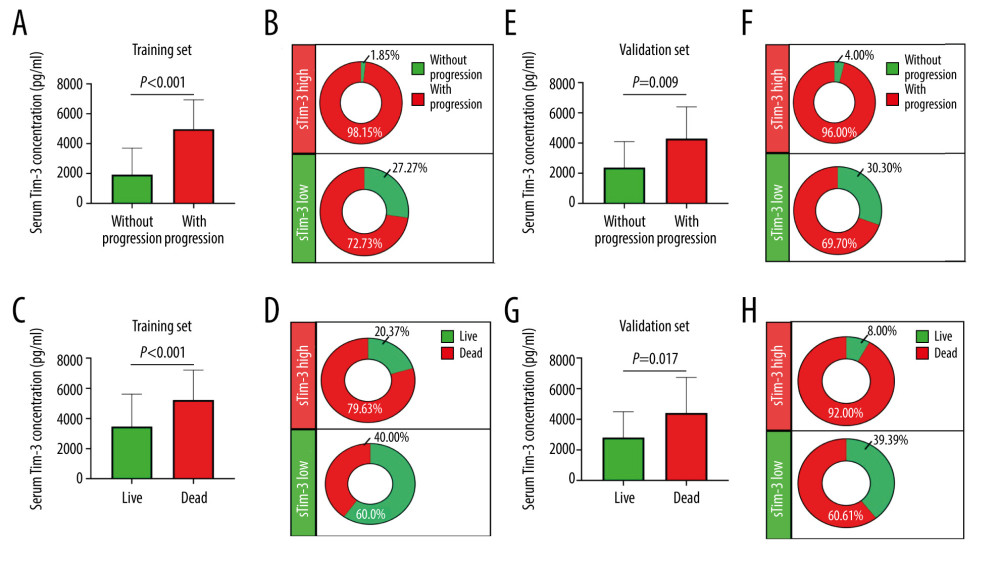 Figure 2. Serum Tim-3 was elevated in HCC patients with poor outcomes. (A) Distribution of serum Tim-3 in HCC patients who had distinct progression after receiving initial TACE treatment, in the training set. (B) Proportion of progression in HCC patients with low and high serum Tim-3 levels after receiving initial TACE treatment, in the training set. (C) Distribution of serum Tim-3 in HCC patients who died vs those who survived after receiving initial TACE treatment, in the training set. (D) Proportion of death in HCC patients with low and high serum Tim-3 levels after receiving initial TACE treatment, in the training set. (E) Distribution of serum Tim-3 in HCC patients who died vs those who survived after receiving initial TACE treatment, in the validation set. (F) Proportion of progression in HCC patients with low and high serum Tim-3 levels after receiving initial TACE treatment, in the validation set. (G) Distribution of serum Tim-3 in HCC patients who died vs those who survived after receiving initial TACE treatment, in the validation set. (H) Proportion of death in HCC patients with low and high serum Tim-3 levels after receiving initial TACE treatment, in the validation set. HCC – hepatocellular carcinoma; sTIM3 – serum Tim-3; TACE – transcatheter arterial chemoembolization; Tim-3 – T cell immunoglobulin and mucin domain 3.
Figure 2. Serum Tim-3 was elevated in HCC patients with poor outcomes. (A) Distribution of serum Tim-3 in HCC patients who had distinct progression after receiving initial TACE treatment, in the training set. (B) Proportion of progression in HCC patients with low and high serum Tim-3 levels after receiving initial TACE treatment, in the training set. (C) Distribution of serum Tim-3 in HCC patients who died vs those who survived after receiving initial TACE treatment, in the training set. (D) Proportion of death in HCC patients with low and high serum Tim-3 levels after receiving initial TACE treatment, in the training set. (E) Distribution of serum Tim-3 in HCC patients who died vs those who survived after receiving initial TACE treatment, in the validation set. (F) Proportion of progression in HCC patients with low and high serum Tim-3 levels after receiving initial TACE treatment, in the validation set. (G) Distribution of serum Tim-3 in HCC patients who died vs those who survived after receiving initial TACE treatment, in the validation set. (H) Proportion of death in HCC patients with low and high serum Tim-3 levels after receiving initial TACE treatment, in the validation set. HCC – hepatocellular carcinoma; sTIM3 – serum Tim-3; TACE – transcatheter arterial chemoembolization; Tim-3 – T cell immunoglobulin and mucin domain 3. 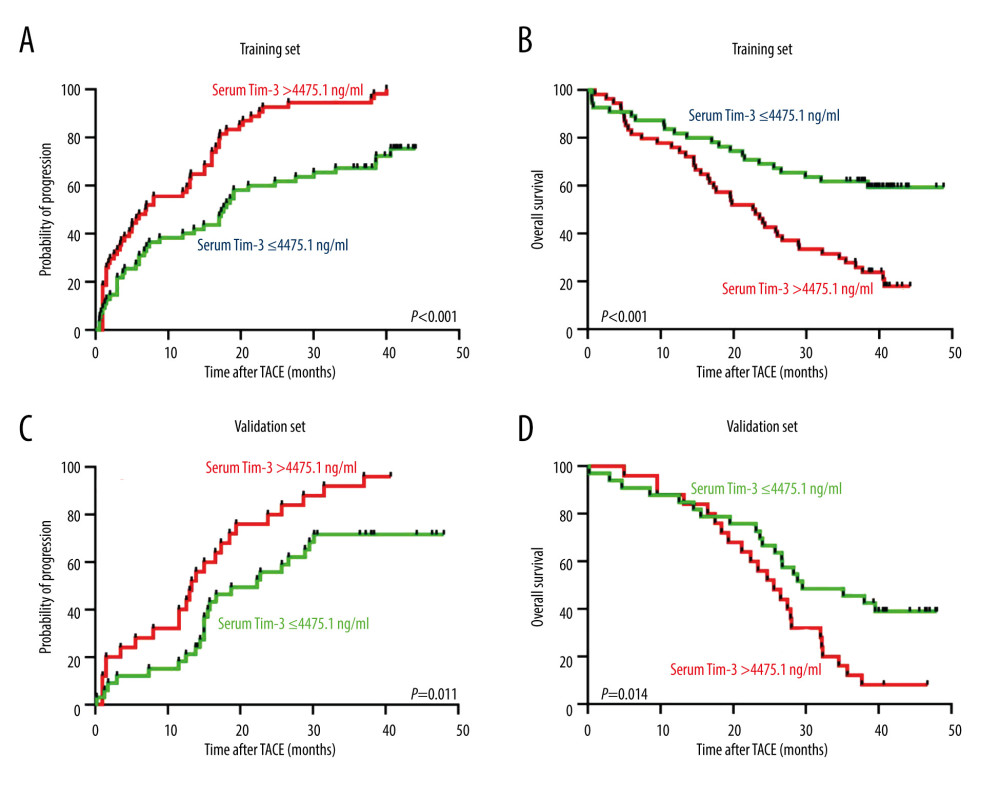 Figure 3. Kaplan-Meier curve analysis of serum Tim-3 in HCC patients receiving TACE treatment. (A) Kaplan-Meier curve analysis for progression, in the training set, when patients were stratified into 2 subgroups according to serum Tim-3 levels. (B) Kaplan-Meier curve analysis for overall survival, in the training set, when patients were stratified into 2 subgroups according to serum Tim-3 levels. (C) Kaplan-Meier curve analysis for progression, in the validation set, when patients were stratified into 2 subgroups according to serum Tim-3 levels. (D) Kaplan-Meier curve analysis for overall survival, in the validation set, when patients were stratified into 2 subgroups according to serum Tim-3 levels. Kaplan-Meier analyses were conducted and log-rank tests were performed. HCC – hepatocellular carcinoma; TACE – transcatheter arterial chemoembolization; Tim-3 – T cell immunoglobulin and mucin domain 3.
Figure 3. Kaplan-Meier curve analysis of serum Tim-3 in HCC patients receiving TACE treatment. (A) Kaplan-Meier curve analysis for progression, in the training set, when patients were stratified into 2 subgroups according to serum Tim-3 levels. (B) Kaplan-Meier curve analysis for overall survival, in the training set, when patients were stratified into 2 subgroups according to serum Tim-3 levels. (C) Kaplan-Meier curve analysis for progression, in the validation set, when patients were stratified into 2 subgroups according to serum Tim-3 levels. (D) Kaplan-Meier curve analysis for overall survival, in the validation set, when patients were stratified into 2 subgroups according to serum Tim-3 levels. Kaplan-Meier analyses were conducted and log-rank tests were performed. HCC – hepatocellular carcinoma; TACE – transcatheter arterial chemoembolization; Tim-3 – T cell immunoglobulin and mucin domain 3. 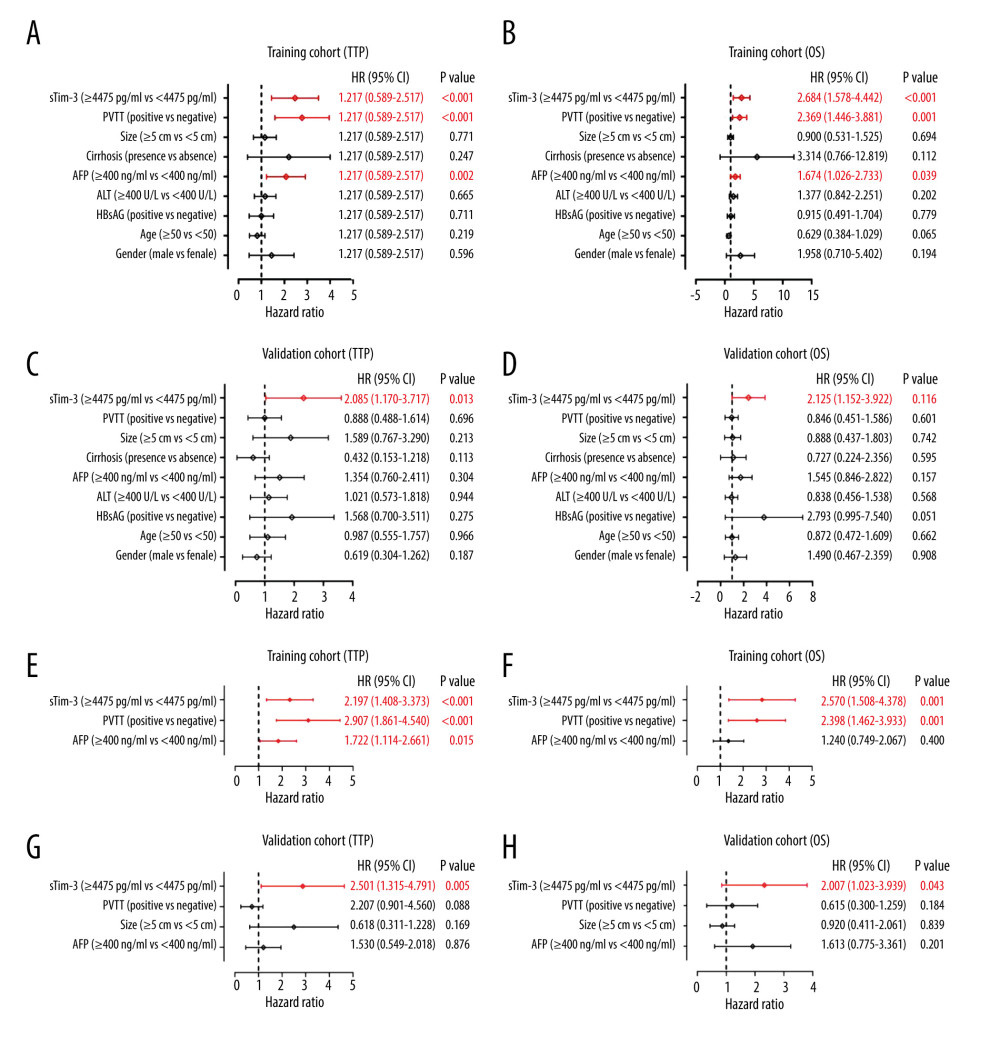 Figure 4. Cox regression analysis of serum Tim-3 in HCC patients receiving TACE treatment. (A) Univariate Cox regression analysis for progression, in the training set, when the patients were stratified into 2 subgroups according to serum Tim-3 levels. (B) Univariate Cox regression analysis for overall survival, in the training set, when the patients were stratified into 2 subgroups according to serum Tim-3 levels. (C) Univariate Cox regression analysis for progression, in the validation set, when the patients were stratified into 2 subgroups according to serum Tim-3 levels. (D) Univariate Cox regression analysis for overall survival, in the validation set, when the patients were stratified into 2 subgroups according to serum Tim-3 levels. (E) Multivariate Cox regression analysis for progression, in the training set, when the patients were stratified into 2 subgroups according to serum Tim-3 levels. (F) Multivariate Cox regression analysis for overall survival, in the training set, when the patients were stratified into 2 subgroups according to serum Tim-3 levels. (G) Multivariate Cox regression analysis for progression, in the validation set, when the patients were stratified into 2 subgroups according to serum Tim-3 levels. (H) Multivariate Cox regression analysis for overall survival, in the validation set, when the patients were stratified into 2 subgroups according to serum Tim-3 levels. HCC – hepatocellular carcinoma; TACE – transcatheter arterial chemoembolization; Tim-3 – T cell immunoglobulin and mucin domain 3.
Figure 4. Cox regression analysis of serum Tim-3 in HCC patients receiving TACE treatment. (A) Univariate Cox regression analysis for progression, in the training set, when the patients were stratified into 2 subgroups according to serum Tim-3 levels. (B) Univariate Cox regression analysis for overall survival, in the training set, when the patients were stratified into 2 subgroups according to serum Tim-3 levels. (C) Univariate Cox regression analysis for progression, in the validation set, when the patients were stratified into 2 subgroups according to serum Tim-3 levels. (D) Univariate Cox regression analysis for overall survival, in the validation set, when the patients were stratified into 2 subgroups according to serum Tim-3 levels. (E) Multivariate Cox regression analysis for progression, in the training set, when the patients were stratified into 2 subgroups according to serum Tim-3 levels. (F) Multivariate Cox regression analysis for overall survival, in the training set, when the patients were stratified into 2 subgroups according to serum Tim-3 levels. (G) Multivariate Cox regression analysis for progression, in the validation set, when the patients were stratified into 2 subgroups according to serum Tim-3 levels. (H) Multivariate Cox regression analysis for overall survival, in the validation set, when the patients were stratified into 2 subgroups according to serum Tim-3 levels. HCC – hepatocellular carcinoma; TACE – transcatheter arterial chemoembolization; Tim-3 – T cell immunoglobulin and mucin domain 3. 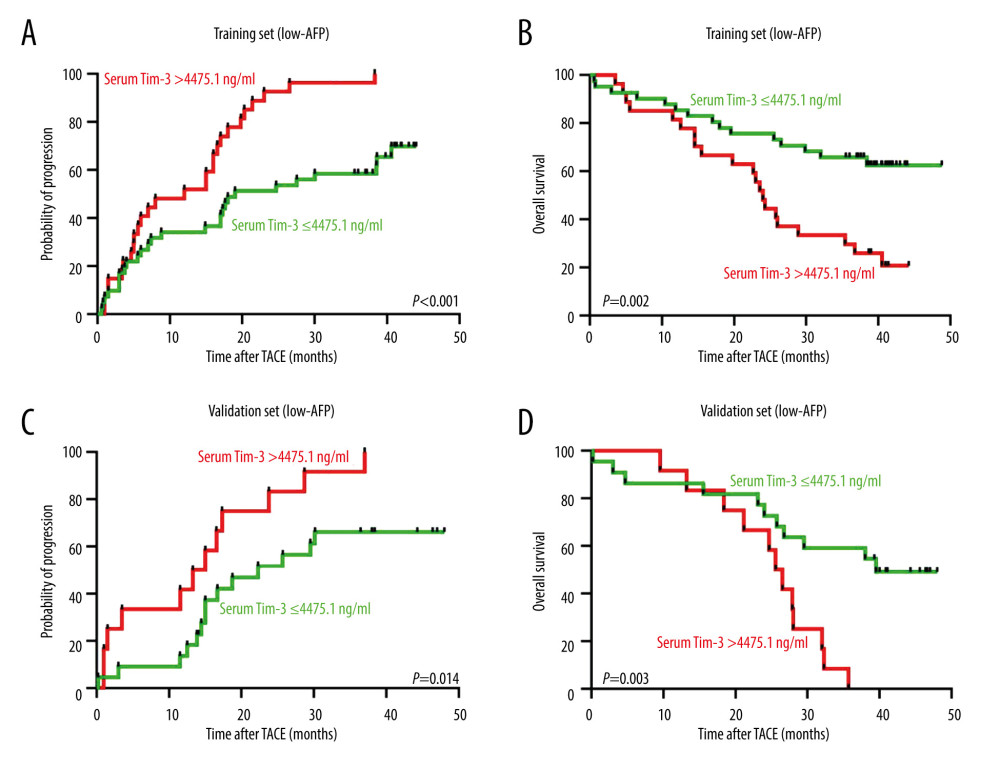 Figure 5. Prognostic value of pre-TACE serum Tim-3 in the low-AFP subgroup. (A) Kaplan-Meier curve analysis for progression, in the training set, when AFP-low patients were stratified into 2 subgroups according to serum Tim-3 levels. (B) Kaplan-Meier curve analysis for death, in the training set, when AFP-low patients were stratified into 2 subgroups according to serum Tim-3 levels. (C) Kaplan-Meier curve analysis for progression, in the validation set, when AFP-low patients were stratified into 2 subgroups according to serum Tim-3 levels. (D) Kaplan-Meier curve analysis for death, in the validation set, when AFP-low patients were stratified into 2 subgroups according to serum Tim-3 levels. Kaplan-Meier analyses were conducted and log-rank tests were performed. AFP – alpha-fetoprotein; TACE – transcatheter arterial chemoembolization; Tim-3 – T cell immunoglobulin and mucin domain 3.
Figure 5. Prognostic value of pre-TACE serum Tim-3 in the low-AFP subgroup. (A) Kaplan-Meier curve analysis for progression, in the training set, when AFP-low patients were stratified into 2 subgroups according to serum Tim-3 levels. (B) Kaplan-Meier curve analysis for death, in the training set, when AFP-low patients were stratified into 2 subgroups according to serum Tim-3 levels. (C) Kaplan-Meier curve analysis for progression, in the validation set, when AFP-low patients were stratified into 2 subgroups according to serum Tim-3 levels. (D) Kaplan-Meier curve analysis for death, in the validation set, when AFP-low patients were stratified into 2 subgroups according to serum Tim-3 levels. Kaplan-Meier analyses were conducted and log-rank tests were performed. AFP – alpha-fetoprotein; TACE – transcatheter arterial chemoembolization; Tim-3 – T cell immunoglobulin and mucin domain 3. 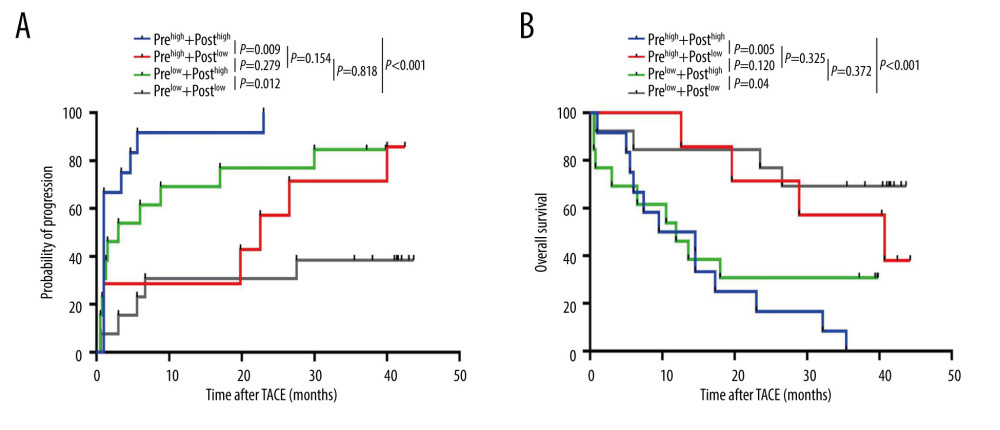 Figure 6. Dynamic changes in serum Tim-3 during the peri-TACE period predicted poor prognosis. (A) Kaplan-Meier curve analysis for progression, in the training set, when patients were stratified according to their peri-TACE period serum Tim-3 levels. (B) Kaplan-Meier curve analysis for death, in the training set, when patients were stratified according to their peri-TACE period serum Tim-3 levels. Kaplan-Meier analyses were conducted and log-rank tests were performed. TACE – transcatheter arterial chemoembolization; Tim-3 – T cell immunoglobulin and mucin domain 3.
Figure 6. Dynamic changes in serum Tim-3 during the peri-TACE period predicted poor prognosis. (A) Kaplan-Meier curve analysis for progression, in the training set, when patients were stratified according to their peri-TACE period serum Tim-3 levels. (B) Kaplan-Meier curve analysis for death, in the training set, when patients were stratified according to their peri-TACE period serum Tim-3 levels. Kaplan-Meier analyses were conducted and log-rank tests were performed. TACE – transcatheter arterial chemoembolization; Tim-3 – T cell immunoglobulin and mucin domain 3. References
1. Choi WM, Yip TCF, Lim YS, Methodological challenges in meta-analysis to assess the risk of hepatocellular carcinoma across chronic hepatitis B treatments: J Hepatol, 2021; 27; 186-94
2. Lee SK, Lee SW, Jang JW, Immunological markers, prognostic factors and challenges following curative treatments for hepatocellular carcinoma: Int J Mol Sci, 2021; 22(19); 10271
3. Llovet JM, Montal R, Villanueva A, Randomized trials and endpoints in advanced HCC: Role of PFS as a surrogate of survival: J Hepatol, 2019; 70(6); 1262-77
4. Esagian SM, Kakos CD, Giorgakis E, Adjuvant transarterial chemoembolization following curative-intent hepatectomy versus hepatectomy alone for hepatocellular carcinoma: A systematic review and meta-analysis of randomized controlled trials: Cancers (Basel), 2021; 13(12); 2984
5. Lencioni R, Chen XP, Dagher L, Treatment of intermediate/advanced hepatocellular carcinoma in the clinic: How can outcomes be improved?: Oncologist, 2010; 15; 42-52
6. Shen Q, Fan J, Yang XR, Serum DKK1 as a protein biomarker for the diagnosis of hepatocellular carcinoma: A large-scale, multicentre study: Lancet Oncol, 2012; 13(8); 817-26
7. Scheiner B, Pomej K, Kristein MM, Prognosis of patients with hepatocellular carcinoma treated with immunotherapy-development and validation of the CRAFITY score: J Hepatol, 2022; 76(2); 353-63
8. Mocan T, Ilies M, Nenu I, Serum levels of soluble programmed death-ligand 1 (sPD-L1): A possible biomarker in predicting post-treatment outcomes in patients with early hepatocellular carcinoma: Int Immuno pharmacol, 2021; 94; 107467
9. Odagiri N, Hai H, Thuy LTT, Early change in the plasma levels of circulating soluble immune checkpoint proteins in patients with unresectable hepatocellular carcinoma treated by lenvatinib or transcatheter arterial chemoembolization: Cancers (Basel), 2020; 12(8); 2045
10. Finkelmeier F, Canli O, Pleli T, High levels of the soluble programmed death-ligand (sPD-L1) identify hepatocellular carcinoma patients with a poor prognosis: Eur J Cancer, 2016; 59; 152-59
11. Acharya N, Sabatos-Peyton C, Anderson AC, Tim-3 finds its place in the cancer immunotherapy landscape: J Immunother Cancer, 2020; 8(1); e000911
12. Wolf Y, Anderson AC, Kuchroo VK, TIM3 comes of age as an inhibitory receptor: Nat Rev Immunol, 2020; 20(3); 173-85
13. Tang R, Rangachari M, Kuchroo VK, Tim-3: A co-receptor with diverse roles in T cell exhaustion and tolerance: Semin Immunol, 2019; 42; 101302
14. Liu F, Liu Y, Chen Z, Tim-3 expression and its role in hepatocellular carcinoma: J Hematol Oncol, 2018; 11(1); 126
15. Ganjalikhani HM, Jafarinia M, Aziz M, The role of TIM-3 in hepatocellular carcinoma: a promising target for immunotherapy?: Front Oncol, 2020; 10; 601661
16. Chen M, Wang L, Wang Y, Soluble Tim3 detection by time-resolved fluorescence immunoassay and its application in membranous nephropathy: J Clin Lab Anal, 2020; 34(6); e23248
17. Tampaki M, Lonas E, Hadziyannis E, Association of TIM-3 with BCLC stage, serum PD-L1 detection, and response to transarterial chemoembolization in patients with hepatocellular carcinoma: Cancers (Basel), 2020; 12(1); 212
18. Li F, Li N, Sang J, Highly elevated soluble Tim-3 levels correlate with increased hepatocellular carcinoma risk and poor survival of hepatocellular carcinoma patients in chronic hepatitis B virus infection: Cancer Manag Res, 2018; 10; 941-51
19. Ma XL, Jiang M, Zhao Y, Application of serum annexin A3 in diagnosis, outcome prediction and therapeutic response evaluation for patients with hepatocellular carcinoma: Ann Surg Oncol, 2018; 25(6); 1686-94
20. Xu Y, Zhang H, Huang Y, Role of TIM-3 in ovarian cancer: Clin Transl Oncol, 2017; 19(9); 1079-83
21. Das M, Zhu C, Kuchroo VK, Tim-3 and its role in regulating anti-tumor immunity: Immunol Rev, 2017; 276(1); 97-111
22. Anderson AC, Tim-3: An emerging target in the cancer immunotherapy landscape: Cancer Immunol Res, 2014; 2(5); 393-98
23. Ren F, Li J, Jiang X, Plasma soluble Tim-3 emerges as an inhibitor in sepsis: Sepsis contrary to membrane Tim-3 on monocytes: Tissue Antigens, 2015; 86(5); 325-32
24. Hansen JA, Hanash SM, Tabellini L, A novel soluble form of Tim-3 associated with severe graft-versus-host disease: Biol Blood Marrow Transplant, 2013; 19(9); 1323-30
25. Feng X, Feng J, Clinical significance of Tim3-positive T cell subsets in patients with multiple sclerosis: J Clin Neurosci, 2016; 34; 193-97
26. Leotta S, Sapienza G, Camuglia MG, Preliminary results of a combined score based on sIL2-Rα and TIM-3 levels assayed early after hematopoietic transplantation: Front Immunol, 2019; 10; 3158
27. Xu B, Yuan L, Gao Q, Circulating and tumor-infiltrating Tim-3 in patients with colorectal cancer: Oncotarget, 2015; 6(24); 20592-603
28. Ge W, Li J, Fan W, Tim-3 as a diagnostic and prognostic biomarker of osteosarcoma: Tumour Biol, 2017; 39(7); 1010428317715643
29. Wang T, Zhang KH, New blood biomarkers for the diagnosis of AFP-negative hepatocellular carcinoma: Front Oncol, 2020; 10; 1316
30. Sauzay C, Petit A, Bourgeois AM, Alpha-foetoprotein (AFP): A multi-purpose marker in hepatocellular carcinoma: Clin Chim Acta, 2016; 463; 39-44
31. Luo P, Wu S, Yu Y, Current status and perspective biomarkers in AFP negative HCC: Towards screening for and diagnosing hepatocellular carcinoma at an earlier stage: Pathol Oncol Res, 2020; 26(2); 599-603
32. Guo W, Yang XR, Sun YF, Clinical significance of EpCAM mRNA-positive circulating tumor cells in hepatocellular carcinoma by an optimized negative enrichment and qRT-PCR-based platform: Clin Cancer Res, 2014; 20(18); 4794-805
Figures
 Figure 1. Serum Tim-3 distribution and correlation with serum AFP level. (A) Distribution of serum Tim-3 in healthy controls, resectable HCC patients, and unresectable HCC patients receiving TACE treatment. (B) Correlation between serum Tim-3 and AFP in the training set. (C) Correlation between serum Tim-3 and AFP in the validation set. AFP – alpha-fetoprotein; HC – healthy controls; HCC – hepatocellular carcinoma; TACE – transcatheter arterial chemoembolization; Tim-3 – T cell immunoglobulin and mucin domain 3.
Figure 1. Serum Tim-3 distribution and correlation with serum AFP level. (A) Distribution of serum Tim-3 in healthy controls, resectable HCC patients, and unresectable HCC patients receiving TACE treatment. (B) Correlation between serum Tim-3 and AFP in the training set. (C) Correlation between serum Tim-3 and AFP in the validation set. AFP – alpha-fetoprotein; HC – healthy controls; HCC – hepatocellular carcinoma; TACE – transcatheter arterial chemoembolization; Tim-3 – T cell immunoglobulin and mucin domain 3. Figure 2. Serum Tim-3 was elevated in HCC patients with poor outcomes. (A) Distribution of serum Tim-3 in HCC patients who had distinct progression after receiving initial TACE treatment, in the training set. (B) Proportion of progression in HCC patients with low and high serum Tim-3 levels after receiving initial TACE treatment, in the training set. (C) Distribution of serum Tim-3 in HCC patients who died vs those who survived after receiving initial TACE treatment, in the training set. (D) Proportion of death in HCC patients with low and high serum Tim-3 levels after receiving initial TACE treatment, in the training set. (E) Distribution of serum Tim-3 in HCC patients who died vs those who survived after receiving initial TACE treatment, in the validation set. (F) Proportion of progression in HCC patients with low and high serum Tim-3 levels after receiving initial TACE treatment, in the validation set. (G) Distribution of serum Tim-3 in HCC patients who died vs those who survived after receiving initial TACE treatment, in the validation set. (H) Proportion of death in HCC patients with low and high serum Tim-3 levels after receiving initial TACE treatment, in the validation set. HCC – hepatocellular carcinoma; sTIM3 – serum Tim-3; TACE – transcatheter arterial chemoembolization; Tim-3 – T cell immunoglobulin and mucin domain 3.
Figure 2. Serum Tim-3 was elevated in HCC patients with poor outcomes. (A) Distribution of serum Tim-3 in HCC patients who had distinct progression after receiving initial TACE treatment, in the training set. (B) Proportion of progression in HCC patients with low and high serum Tim-3 levels after receiving initial TACE treatment, in the training set. (C) Distribution of serum Tim-3 in HCC patients who died vs those who survived after receiving initial TACE treatment, in the training set. (D) Proportion of death in HCC patients with low and high serum Tim-3 levels after receiving initial TACE treatment, in the training set. (E) Distribution of serum Tim-3 in HCC patients who died vs those who survived after receiving initial TACE treatment, in the validation set. (F) Proportion of progression in HCC patients with low and high serum Tim-3 levels after receiving initial TACE treatment, in the validation set. (G) Distribution of serum Tim-3 in HCC patients who died vs those who survived after receiving initial TACE treatment, in the validation set. (H) Proportion of death in HCC patients with low and high serum Tim-3 levels after receiving initial TACE treatment, in the validation set. HCC – hepatocellular carcinoma; sTIM3 – serum Tim-3; TACE – transcatheter arterial chemoembolization; Tim-3 – T cell immunoglobulin and mucin domain 3. Figure 3. Kaplan-Meier curve analysis of serum Tim-3 in HCC patients receiving TACE treatment. (A) Kaplan-Meier curve analysis for progression, in the training set, when patients were stratified into 2 subgroups according to serum Tim-3 levels. (B) Kaplan-Meier curve analysis for overall survival, in the training set, when patients were stratified into 2 subgroups according to serum Tim-3 levels. (C) Kaplan-Meier curve analysis for progression, in the validation set, when patients were stratified into 2 subgroups according to serum Tim-3 levels. (D) Kaplan-Meier curve analysis for overall survival, in the validation set, when patients were stratified into 2 subgroups according to serum Tim-3 levels. Kaplan-Meier analyses were conducted and log-rank tests were performed. HCC – hepatocellular carcinoma; TACE – transcatheter arterial chemoembolization; Tim-3 – T cell immunoglobulin and mucin domain 3.
Figure 3. Kaplan-Meier curve analysis of serum Tim-3 in HCC patients receiving TACE treatment. (A) Kaplan-Meier curve analysis for progression, in the training set, when patients were stratified into 2 subgroups according to serum Tim-3 levels. (B) Kaplan-Meier curve analysis for overall survival, in the training set, when patients were stratified into 2 subgroups according to serum Tim-3 levels. (C) Kaplan-Meier curve analysis for progression, in the validation set, when patients were stratified into 2 subgroups according to serum Tim-3 levels. (D) Kaplan-Meier curve analysis for overall survival, in the validation set, when patients were stratified into 2 subgroups according to serum Tim-3 levels. Kaplan-Meier analyses were conducted and log-rank tests were performed. HCC – hepatocellular carcinoma; TACE – transcatheter arterial chemoembolization; Tim-3 – T cell immunoglobulin and mucin domain 3. Figure 4. Cox regression analysis of serum Tim-3 in HCC patients receiving TACE treatment. (A) Univariate Cox regression analysis for progression, in the training set, when the patients were stratified into 2 subgroups according to serum Tim-3 levels. (B) Univariate Cox regression analysis for overall survival, in the training set, when the patients were stratified into 2 subgroups according to serum Tim-3 levels. (C) Univariate Cox regression analysis for progression, in the validation set, when the patients were stratified into 2 subgroups according to serum Tim-3 levels. (D) Univariate Cox regression analysis for overall survival, in the validation set, when the patients were stratified into 2 subgroups according to serum Tim-3 levels. (E) Multivariate Cox regression analysis for progression, in the training set, when the patients were stratified into 2 subgroups according to serum Tim-3 levels. (F) Multivariate Cox regression analysis for overall survival, in the training set, when the patients were stratified into 2 subgroups according to serum Tim-3 levels. (G) Multivariate Cox regression analysis for progression, in the validation set, when the patients were stratified into 2 subgroups according to serum Tim-3 levels. (H) Multivariate Cox regression analysis for overall survival, in the validation set, when the patients were stratified into 2 subgroups according to serum Tim-3 levels. HCC – hepatocellular carcinoma; TACE – transcatheter arterial chemoembolization; Tim-3 – T cell immunoglobulin and mucin domain 3.
Figure 4. Cox regression analysis of serum Tim-3 in HCC patients receiving TACE treatment. (A) Univariate Cox regression analysis for progression, in the training set, when the patients were stratified into 2 subgroups according to serum Tim-3 levels. (B) Univariate Cox regression analysis for overall survival, in the training set, when the patients were stratified into 2 subgroups according to serum Tim-3 levels. (C) Univariate Cox regression analysis for progression, in the validation set, when the patients were stratified into 2 subgroups according to serum Tim-3 levels. (D) Univariate Cox regression analysis for overall survival, in the validation set, when the patients were stratified into 2 subgroups according to serum Tim-3 levels. (E) Multivariate Cox regression analysis for progression, in the training set, when the patients were stratified into 2 subgroups according to serum Tim-3 levels. (F) Multivariate Cox regression analysis for overall survival, in the training set, when the patients were stratified into 2 subgroups according to serum Tim-3 levels. (G) Multivariate Cox regression analysis for progression, in the validation set, when the patients were stratified into 2 subgroups according to serum Tim-3 levels. (H) Multivariate Cox regression analysis for overall survival, in the validation set, when the patients were stratified into 2 subgroups according to serum Tim-3 levels. HCC – hepatocellular carcinoma; TACE – transcatheter arterial chemoembolization; Tim-3 – T cell immunoglobulin and mucin domain 3. Figure 5. Prognostic value of pre-TACE serum Tim-3 in the low-AFP subgroup. (A) Kaplan-Meier curve analysis for progression, in the training set, when AFP-low patients were stratified into 2 subgroups according to serum Tim-3 levels. (B) Kaplan-Meier curve analysis for death, in the training set, when AFP-low patients were stratified into 2 subgroups according to serum Tim-3 levels. (C) Kaplan-Meier curve analysis for progression, in the validation set, when AFP-low patients were stratified into 2 subgroups according to serum Tim-3 levels. (D) Kaplan-Meier curve analysis for death, in the validation set, when AFP-low patients were stratified into 2 subgroups according to serum Tim-3 levels. Kaplan-Meier analyses were conducted and log-rank tests were performed. AFP – alpha-fetoprotein; TACE – transcatheter arterial chemoembolization; Tim-3 – T cell immunoglobulin and mucin domain 3.
Figure 5. Prognostic value of pre-TACE serum Tim-3 in the low-AFP subgroup. (A) Kaplan-Meier curve analysis for progression, in the training set, when AFP-low patients were stratified into 2 subgroups according to serum Tim-3 levels. (B) Kaplan-Meier curve analysis for death, in the training set, when AFP-low patients were stratified into 2 subgroups according to serum Tim-3 levels. (C) Kaplan-Meier curve analysis for progression, in the validation set, when AFP-low patients were stratified into 2 subgroups according to serum Tim-3 levels. (D) Kaplan-Meier curve analysis for death, in the validation set, when AFP-low patients were stratified into 2 subgroups according to serum Tim-3 levels. Kaplan-Meier analyses were conducted and log-rank tests were performed. AFP – alpha-fetoprotein; TACE – transcatheter arterial chemoembolization; Tim-3 – T cell immunoglobulin and mucin domain 3. Figure 6. Dynamic changes in serum Tim-3 during the peri-TACE period predicted poor prognosis. (A) Kaplan-Meier curve analysis for progression, in the training set, when patients were stratified according to their peri-TACE period serum Tim-3 levels. (B) Kaplan-Meier curve analysis for death, in the training set, when patients were stratified according to their peri-TACE period serum Tim-3 levels. Kaplan-Meier analyses were conducted and log-rank tests were performed. TACE – transcatheter arterial chemoembolization; Tim-3 – T cell immunoglobulin and mucin domain 3.
Figure 6. Dynamic changes in serum Tim-3 during the peri-TACE period predicted poor prognosis. (A) Kaplan-Meier curve analysis for progression, in the training set, when patients were stratified according to their peri-TACE period serum Tim-3 levels. (B) Kaplan-Meier curve analysis for death, in the training set, when patients were stratified according to their peri-TACE period serum Tim-3 levels. Kaplan-Meier analyses were conducted and log-rank tests were performed. TACE – transcatheter arterial chemoembolization; Tim-3 – T cell immunoglobulin and mucin domain 3. In Press
05 Mar 2024 : Clinical Research
Role of Critical Shoulder Angle in Degenerative Type Rotator Cuff Tears: A Turkish Cohort StudyMed Sci Monit In Press; DOI: 10.12659/MSM.943703
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952