03 June 2022: Clinical Research
Early Enteral Nutrition Can Reduce Incidence of Postoperative Hydrocephalus in Patients with Severe Hypertensive Intracerebral Hemorrhage
Zhi Cai1BCE, Kai Zhao1AEG, Yu Li1BC, Xueyan Wan1B, Chunlin Li1B, Hongquan Niu1DF, Kai Shu1DF, Ting Lei1A*DOI: 10.12659/MSM.935850
Med Sci Monit 2022; 28:e935850
Abstract
BACKGROUND: Hydrocephalus secondary to hypertensive intracerebral hemorrhage (HICH) dramatically affects the prognosis. Early enteral nutrition (EN) is beneficial to severe HICH patients, but the impact of early EN administration on hydrocephalus remains unknown. This study aimed to explore the predictors for hydrocephalus occurrence after HICH, with special focus on the effect of early EN application.
MATERIAL AND METHODS: We retrospectively analyzed 146 patients with severe HICH who underwent microsurgery between January 2014 and October 2019 in our department. Patients were divided into early EN (≤48 h) and delayed EN (>48 h) group according to the time-point of EN administration. The diagnosis of hydrocephalus was confirmed by both radiological evaluation and an Evan index method. Diagnosis confirmed within 2 weeks after HICH was identified as acute hydrocephalus, otherwise, it was considered as chronic hydrocephalus.
RESULTS: Twenty-seven patients experienced acute hydrocephalus, while 20 patients developed chronic hydrocephalus. Low preoperative Glasgow coma scale (GCS), subarachnoid hemorrhage (SAH), intraventricular hemorrhage (IVH), delayed EN administration, high levels of postoperative white blood cell, neutrophil, neutrophil-to-lymphocyte ratio, C-reactive protein (CRP), and lactate dehydrogenase were positively related to the occurrence of chronic hydrocephalus (p<0.05), while only IVH was correlated with acute hydrocephalus occurrence (p<0.05). In addition, a multivariate analysis demonstrated that preoperative GCS, SAH, IVH, and early EN administration (p<0.05) were independent predictors for chronic hydrocephalus occurrence.
CONCLUSIONS: Early EN administration, SAH, IVH, and preoperative GCS were associated with the occurrence of chronic hydrocephalus in severe HICH patients. Early EN administration may inhibit the inflammatory response of brain-gut axis, which in turn reduces chronic hydrocephalus occurrence.
Keywords: Cerebral Hemorrhage, Enteral Nutrition, Hydrocephalus, Risk Factors, Humans, Incidence, Intracranial Hemorrhage, Hypertensive, Subarachnoid Hemorrhage
Background
Hypertensive intracranial hemorrhage (HICH) is a severe complication of primary hypertension, which raises many problems, not only patient related to prognosis but also to medical care and public health burdens [1–3]. Previous studies have indicated that 58% of patients with intracranial hemorrhage (ICH) survived less than 1 year, while 2/3 of the survivors had severe disabilities [4]. Secondary hydrocephalus is one of the most common complications of HICH, and is positively associated with patient poor prognosis [5]. Although cerebrospinal fluid (CSF) shunt surgery for hydrocephalus exerts a good curative effect in most patients, various related complications, such as intracranial infection, blockage of shunt canal, and slit ventricle syndrome, can be disastrous [6]. Increasing data demonstrate that various factors are related to the incidence of secondary hydrocephalus, including age, Glasgow coma scale (GCS), intraventricular hemorrhage (IVH), decompressive craniectomy (DC) and inflammation [7–10]. Targeting these factors reduced the prevalence of secondary hydrocephalus in many patients. For instance, the CSF replacement can decrease hydrocephalus occurrence, while reducing bone flap area of DC most likely has the same effect [11]. Inflammatory response plays a pivotal role in regulation of CSF secretion, circulation, and absorption, which was widely investigated recently [8]. Preclinical experiments revealed that inflammatory factors, such as interleukin-6 (IL-6), interleukin-8 (IL-8), and tumor growth factor β (TGF-β) in CSF and in peripheral blood, were associated with hydrocephalus [8,12]. More intriguingly, clinical application of dexamethasone in subarachnoid hemorrhage (SAH) patients inhibited the inflammatory response and was associated with a lower incidence of hydrocephalus [13]. These exciting clinical findings suggest that inhibition of inflammation can decrease hydrocephalus occurrence, which is important for developing novel treatment strategies for HICH patients.
Patients with HICH always experience chronic consciousness disturbance, which leads to multiple complications such as hypostatic pneumonia, deep venous thrombosis, and multiple organ dysfunctions. Moreover, HICH patients can develop a high catabolism, which results in obvious weight loss and insufficient nutrition supply [14]. Accumulating evidence has demonstrated that nutrition support plays crucial roles in critical ill patients, especially in severe neurological patients. Enteral nutrition (EN) administration is considered as an optimal feeding approach for patients without gastrointestinal dysfunction [15]. EN administration not only provides enough nutrition, but also improves the protein and energy intake though prevention of stress to the gastrointestinal tract [16]. Additionally, application of EN potentially decreases bacterial translocation, reduces inflammatory response, and even regulates brain function through a brain-gut axis [17–19]. EN can markedly relieve the perioperative inflammatory responses, improve immunity, and maintain intestinal flora structure [20]. Some encouraging studies even showed that early application of EN within 48 h after injury could achieve a better curative effect [21]. In traumatic brain injury patients, increased caloric supply was associated with a decrease in mortality and in-hospital complications [22].
However, whether application of early EN influences the hydrocephalus occurrence is unclear. To this end, our current retrospective study aimed to investigate the potential roles of early EN on hydrocephalus occurrence in severe HICH patients and to discover the underlying mechanism.
Material and Methods
PATIENT POPULATION AND STUDY DESIGN:
We retrospectively reviewed the clinical data of patients with acute ICH who underwent surgical treatments in our department between January 2014 and October 2019.
The inclusion criteria were: (1) age ≥18 years; (2) ICH was identified by CT scan in 24 h after onset; (3) Patients had hypertension history or with hypertension in 3 separate sphygmomanometer measurements during the admission period (systolic pressure ≥140 mmHg or diastolic pressure ≥90 mmHg) combined with evidence of end-stage organ injury [23]; (4) Patients in coma (GCS score <8), midline shift, large hematomas, or patients with refractory intracranial pressure received DC. Patients with a hematoma diameter ≥2 cm and GCS ≥5 require hematoma removal, and patients with acute hydrocephalus due to IVH or large intraparenchymal hematomas with mass effect associated with impaired level of consciousness (ie, GCS ≤8) may require the urgent placement of an EVD, all these operations were performed by experienced neurosurgeons [24,25], and EN administration in neurosurgical intensive unit for at least 2 weeks; and (5) GCS ≤12 postoperatively.
The exclusion criteria were: (1) ICH secondary to cerebral trauma, intracranial tumor, specific cerebrovascular diseases (intracranial ruptured aneurysms, arteriovenous malformations and moyamoya disease), abnormal brain structures or administration of anticoagulant drug; (2) EN administration was started at the time-point >7 days postoperatively; (3) Patients were identified as intracranial infection during hospitalization; and (4) Lost to follow-up or died. Patients enrolled in this study were treated in our neurosurgical intensive unit for at least 2 weeks with optimal therapy after the operation, and the nutrition therapy was adjusted daily according to the monitoring of nutritional status and metabolic requirement in each patient, especially for standard EN treatment.
Study procedures involving human participants were in accordance with the Helsinki Declaration. The Medical Ethics Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology approved this study.
DATA COLLECTION:
Nutritional status was evaluated by body mass index (BMI), mid-arm muscle circumference, skinfold thickness, the level of serum albumin, the score of nutrition risk screening (NRS2002), and scored subjective global Assessment (SGA) at admission and at 2 weeks after surgery. Nutrition therapy was performed in patients who met the condition of scale ≥3 in NRS2002 [26,27]. The volume of BMI was measured by a formula of BMI=body weight/height2 [28]. EN therapy was recommended in HICH patients without contraindication to enteral feeding in priority. We divided patients into 2 groups according to the time-point of EN administration, which was preferred in 48 h after surgery (early EN group) and delayed in patients under the conditions of elevated gastric residual volumes (>400 mL), transient upper gastrointestinal bleeding, abdominal distention, and emesis (delayed EN group). The EN administration was performed for all patients according to previous guidelines [29,30]. Briefly, patients were initially started on EN at 20 mL/h by nasointestinal tube and advanced by 20 mL in the next 24 h until the nutrition goal was reached, which included energy goal at 20–25 kcal/kg of body weight per day and protein goal at 1.5–2.5 g/kg of body weight per day [29]. These targets in each patient should be gradually achieved within 2–3 days and maintained for at least 2 weeks [29].
The clinical data including the general condition, such as age, sex, GCS evaluation, radiological images, laboratory examinations, surgical strategy, and hydrocephalus evaluation were collected from medical records. Cranial computed tomography (CT) was performed in all patients. The location of hematoma, SAH, and IVH were determined from CT images by experienced neurosurgeons. The volume of hematoma was directly measured on CT images using a formula of ½A×B×C. “A” and “B” represent the longest distance through the hematoma respectively, while “C” represents the thickness of the hematoma [31].
Diagnose of hydrocephalus was identified by the following standards: (1) Evans index >0.3 (the ratio of the greatest distance between bilateral anterior horns of lateral ventricles and the greatest internal distance of the skull) (Figure 1); and (2) enlarge of the anterior horns of lateral ventricles, temporal horns and the third ventricle accompanied with periventricular cerebral edema [32]. Identification of hydrocephalus at time-point within 2 weeks and after 2 weeks postoperatively was considered as acute and chronic hydrocephalus, respectively. The outcome was evaluated by modified Rankin scale (mRS). The follow-up was performed from discharge to 3 months postoperatively by outpatient visit or by internet video interview. In addition, we collected blood specimens for laboratory examination from each patient at admission, every 2–3 days 2 weeks postoperatively. Extra blood tests were performed individually according to state of the illness. The values of each laboratory results were evaluated as the extremum.
STATISTICAL ANALYSIS:
All statistical analysis were performed by SPSS23.0 software. Normal testing was performed for all data before comparative analysis. The independent
Results
Initial screening of clinical records identified 228 cases who met the inclusion criteria. Of them, 82 patients were excluded due to the exclusion criteria. As a result, a total of 146 patients with severe HICH were enrolled in the present study. The clinical characteristics of patients are summarized and compared in Tables 1 and 2. There were 87 males (59.6%) and 59 females (40.4%), with an average age of 51.7±11.4 years. The average preoperative GCS score was 6.2 (5–8); 103 patients (70.5%) underwent surgical treatment for hematoma removal, while 45 patients (34.9%) received external EVD, and 28 patients (15.0%) also received DC. Intracranial hematomas were located in various lobes in 36 patients (24.6%), basal ganglia in 81 patients (55.5%), thalamus in 15 patients (10.3%), and cerebellum in 14 patients (9.6%). Moreover, IVH was observed in 84 patients (57.5%) while SAH was found in 25 patients (17.1%). The initial nutritional status was evaluated at admission. BMI in patients was calculated as 24.1±3.5 Kg/m2; mid-arm muscle circumference was 30.1±4.5 cm, and the level of serum albumin was 42.4(39.3–47.4) g/L. The mean postoperative GCS score was 10.4 (7–14), and mRS score was 4.2±0.4. Fifty-one patients (34.9%) received early EN treatment, while 95 patients (65.1%) underwent delayed EN application, and 87 patients (59.6%) received supported parenteral nutrition (PN) administration. To assess whether EN administration achieved the treatment goals, the nutritional status was evaluated at 2 weeks after the operation and described as follows: BMI 23.0±3.0 Kg/m2; mid-arm muscle circumference was 28.6±3.9 cm and the level of serum albumin was 33.6±4.9 g/L. Moreover, 27 patients (18.5%) experienced acute hydrocephalus, while 20 patients (13.7%) developed chronic hydrocephalus, and 12 patients (8.2%) had VP shunt surgery.
Clinical variables such as sex, age, nutritional status at admission and at 2 weeks postoperatively, hematoma location, hematoma volume, different surgical treatment, duration from onset to surgery, and postoperative pulmonary infection were not correlated with acute or chronic hydrocephalus occurrence. The incidence of acute hydrocephalus in patients with administration of early EN and delayed EN were 22.1% and 11.8%, respectively, and the incidence of chronic hydrocephalus was 6.7% and 23.0%. More intriguingly, in comparison to delayed EN, early EN reduced the occurrence of chronic hydrocephalus (
Various laboratory parameters were also collected and comparatively analyzed (Tables 2, 3). In particular, univariate regression analysis demonstrated that high postoperative levels of lactate white blood cells (WBC), neutrophil, neutrophil-to-lymphocyte ratio (NLR), C-reactive protein (CRP), and dehydrogenase (LDH) were positively correlated with chronic hydrocephalus occurrence (
In addition, we also explored the impact of EN administration on various laboratory parameters (Table 4). Significantly lower levels of WBC, neutrophil, NLR, and LDH were detected in the early EN group compared to those in the delayed EN group (
Discussion
In summary, our study showed that the early EN administration could reduce the incidence of postoperative hydrocephalus in patients with severe HICH. Early EN administration, preoperative GCS, SAH, and IVH were independent predictors for chronic hydrocephalus occurrence.
Hydrocephalus is a common complication after HICH and can lead to neurological abnormalities. The incidence of hydrocephalus after intracerebral hemorrhage is reported as 10–20% [7,33]. In our study, the incidence was 18.5% for acute hydrocephalus and 13.7% for chronic hydrocephalus. Numerous clinical features revealed potential relationships with the prevalence of hydrocephalus in HICH patients. In general, a lower GCS score can be used for predicting hydrocephalus morbidity after ICH [34]. HICH patients with IVH displayed a higher morbidity of hydrocephalus, and IVH is an independent factor of poor prognosis [35]. DC treatment in patients influenced the CSF circulation and absorption, which in turn resulted in development of chronic hydrocephalus [36]. Our study revealed that low GCS score, SAH, and IVH were related to chronic hydrocephalus occurrence, but only IVH influenced acute hydrocephalus occurrence, which agrees with the literature. Moreover, we found that early application of EN contributed to chronic hydrocephalus occurrence, which was a result of increased intracranial pressure (ICP). Elevated ICP was associated with reduced enteral feeding tolerance, which might explain the lower use of early EN in patients with hydrocephalus. In addition, the systematic inflammation was also highly involved in hydrocephalus generation and development. Preclinical experiments indicated the inflammatory response mediated by Toll-Like Receptor 4 (TLR4) signaling in mouse hydrocephalus models [8,37]. Further investigation determined the inflammatory cells infiltration in choroid plexus and subependymal zone in hydrocephalus. Moreover, clinical studies revealed that inflammatory factors, including IL-6, IL-8, and TNF-α, were significantly associated with the development of hydrocephalus in patients [38,39]. Furthermore, the application of TLR4 inhibitor in mice reduced the inflammatory cells infiltration and inflammatory factors release, which in turn reversed ventriculomegaly. Clinical application of dexamethasone in SAH patients inhibited the inflammatory response and was associated with a lower incidence of hydrocephalus [13]. These facts suggested that targeting the inflammatory response might reduce hydrocephalus occurrence. Our data show that early EN reduced the expression of inflammatory factors (IL-1β and TNF-α) in the blood, which in turn reduced chronic hydrocephalus occurrence [40–42].
HICH patients often experience gastrointestinal injury and immune system disturbance [21]. The gastrointestinal injury usually results in intestine barrier dysfunction, gut microbiotic dysbiosis, and bacteria translocation. The brain-gut axis has been identified for decades and has received the most research attention to date [43]. Through this neuroendocrine pathway, dysfunctions of gut microbiota affect various neurological diseases, such as multiple sclerosis, Parkinson disease, Alzheimer disease, infantile autism, depressive disorder, epilepsy, cerebral stroke, and brain injury [44]. However, the underlying mechanism remains unclear. Gut microbiota dysfunction might influence the transportation of T cells toward the brain, which in turn affects the inflammatory response and neurological function in patients with cerebral stroke [44]. Moreover, gut microbiota dysfunction leads to short-chain fatty acid productive anomaly, which triggers immunological change through the regulation of microglia function [45]. EN administration, especially early application of EN, is usually performed in severe neurological patients, including severe HICH patients. Application of EN treatment protects the gastrointestinal tract from stress and injury, regulates gut microbiota, and decreases bacterial translocation. Increasing data reveal that providing early EN, rather than delayed EN, to critically ill patients reduces inflammation and improves patient prognosis [16,18,46,47]. Early enteral nutrition provides benefits to critically ill patients. Enteral nutrition directly stimulates intestinal peristalsis and the release of trophic substances and neuropeptides, which play an important role in mucosal defenses [48]. Enteral nutrition stimulates the release of immunoglobulin A (IgA) by gut-associated lymphoid tissues, thereby reducing the adhesion of bacteria to epithelial cells and preventing the increase in intestinal permeability [49]. Our study found that the counts of WBC, neutrophil, NLR, CRP, and LDH were significantly associated with chronic hydrocephalus occurrence, indicating the potential role of systemic inflammation in chronic hydrocephalus. Chen analyzed 316 patients with severe brain injury and found that NLR could predict the long-term efficacy of severe brain injury with a sensitivity of 74.3% and specificity of 72.9%. LDH was also a biomarker for inflammatory activity, which was increased in CSF and displayed a relationship with hydrocephalus after ICH [50]. Further study revealed that early EN reduced intestinal permeability, which decreased bacterial colony transmission and inhibited the systemic inflammation, thus reducing the incidence rate of chronic hydrocephalus. The information discussed above suggested to us that early EN reduced chronic hydrocephalus occurrence, potentially through the inhibition of inflammation. Therefore, additional research is needed focusing on the gut microbiotas which produce metabolites with the effects on inhibition of inflammatory response systemically and in central nerve system, which might provide a novel treatment strategy for hydrocephalus secondary to HICH.
Our study provides novel insights into the mechanisms and management strategies of secondary injury after HICH. However, there were several limitations which need to be addressed in the future. First of all, this was a retrospective study performed at a single center. The number of patients involved in this study was relatively small due to the rigorous inclusion and exclusion criteria. Secondly, EN administration in each patient was normalized in accordance to the standard procedure and the individual nutrition targets. Finally, the follow-up period was not long enough, which might have missed patients who developed hydrocephalus after 3 months. The inflammatory factors were not completely detected in peripheral blood and in CSF. To fill this gap, a randomized, controlled, multi-center clinical trial is needed.
Conclusions
Early EN application, SAH, IVH and preoperative GCS are associated with the prevalence of chronic hydrocephalus in severe HICH patients. Early EN administration inhibited the inflammatory response of the brain-gut axis, which in turn reduced chronic hydrocephalus occurrence.
Figures
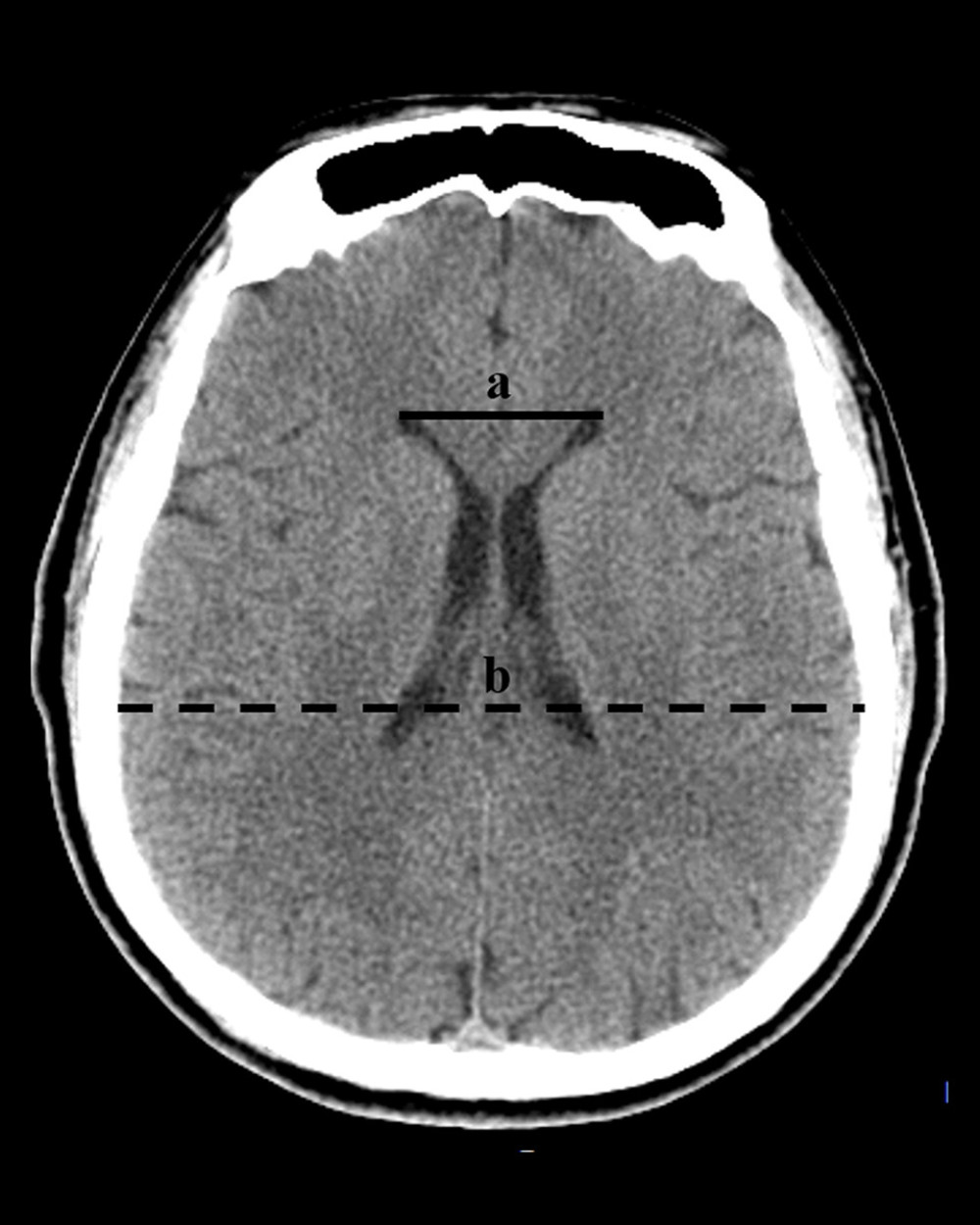 Figure 1. The diagnosis of hydrocephalus was performed by using an Evan index method. The distance between the frontal horns of the lateral ventricles (full line indicated with ‘a’) and the longest inner diameter of skull at the same plane (dotted line indicated with ‘b’). The Evans index was calculated as the ratio of a to b. (Adobe Photoshop CC, 14.0, Adobe).
Figure 1. The diagnosis of hydrocephalus was performed by using an Evan index method. The distance between the frontal horns of the lateral ventricles (full line indicated with ‘a’) and the longest inner diameter of skull at the same plane (dotted line indicated with ‘b’). The Evans index was calculated as the ratio of a to b. (Adobe Photoshop CC, 14.0, Adobe). 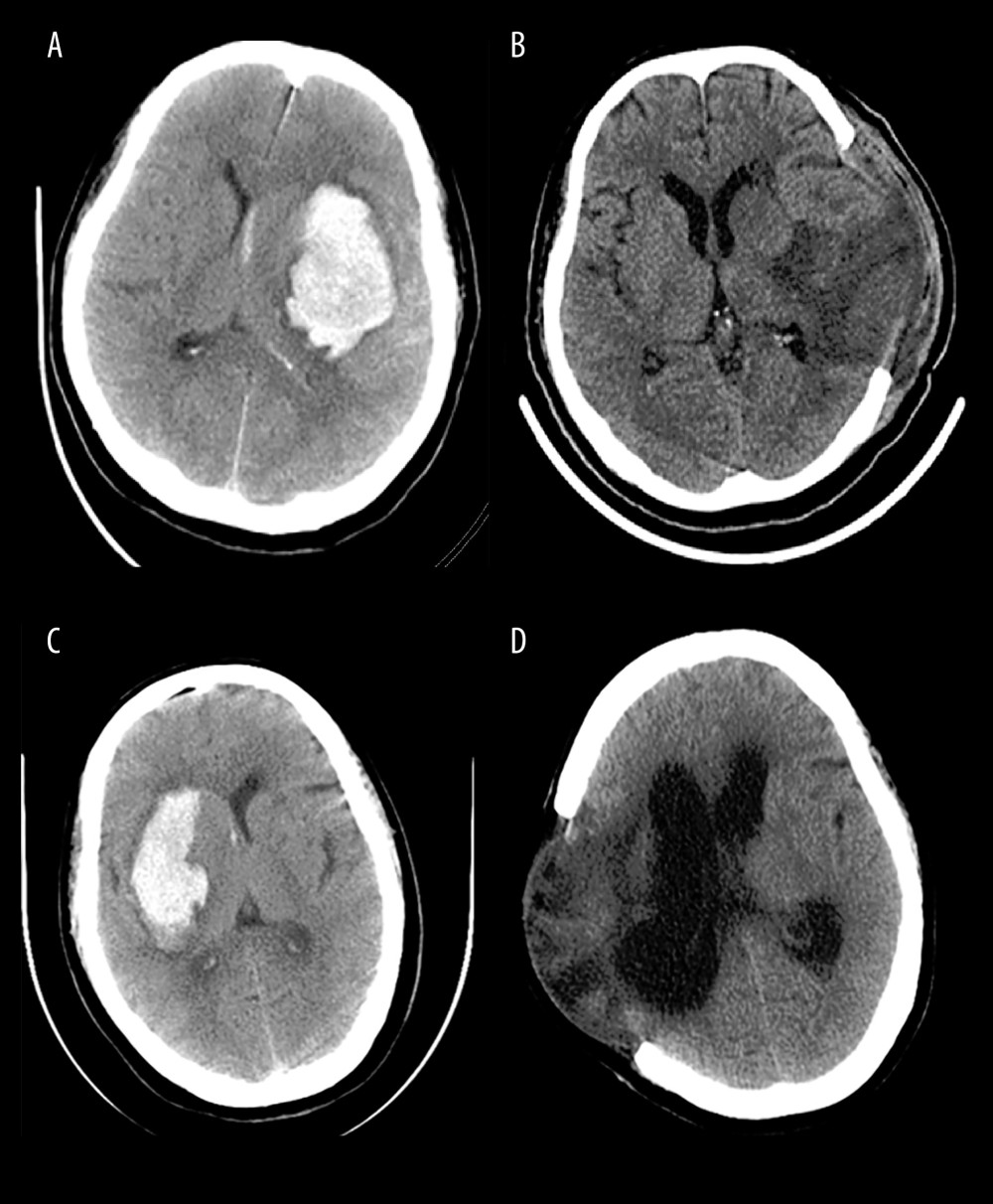 Figure 2. Representative CT images from HICH patients. The patients with severe HICH underwent microsurgery for hematoma removal followed decompression craniectomy received early EN (A, B) and delayed EN (C, D) treatment, respectively. CT images were acquired at admission (A, C) and at 3 months (B, D) postoperatively. Patient with early EN did not develop hydrocephalus, while patient with delayed EN developed chronic hydrocephalus during follow-up. (Adobe Photoshop CC, 14.0, Adobe).
Figure 2. Representative CT images from HICH patients. The patients with severe HICH underwent microsurgery for hematoma removal followed decompression craniectomy received early EN (A, B) and delayed EN (C, D) treatment, respectively. CT images were acquired at admission (A, C) and at 3 months (B, D) postoperatively. Patient with early EN did not develop hydrocephalus, while patient with delayed EN developed chronic hydrocephalus during follow-up. (Adobe Photoshop CC, 14.0, Adobe). Tables
Table 1. The clinical characteristics of 146 patients with severe HICH.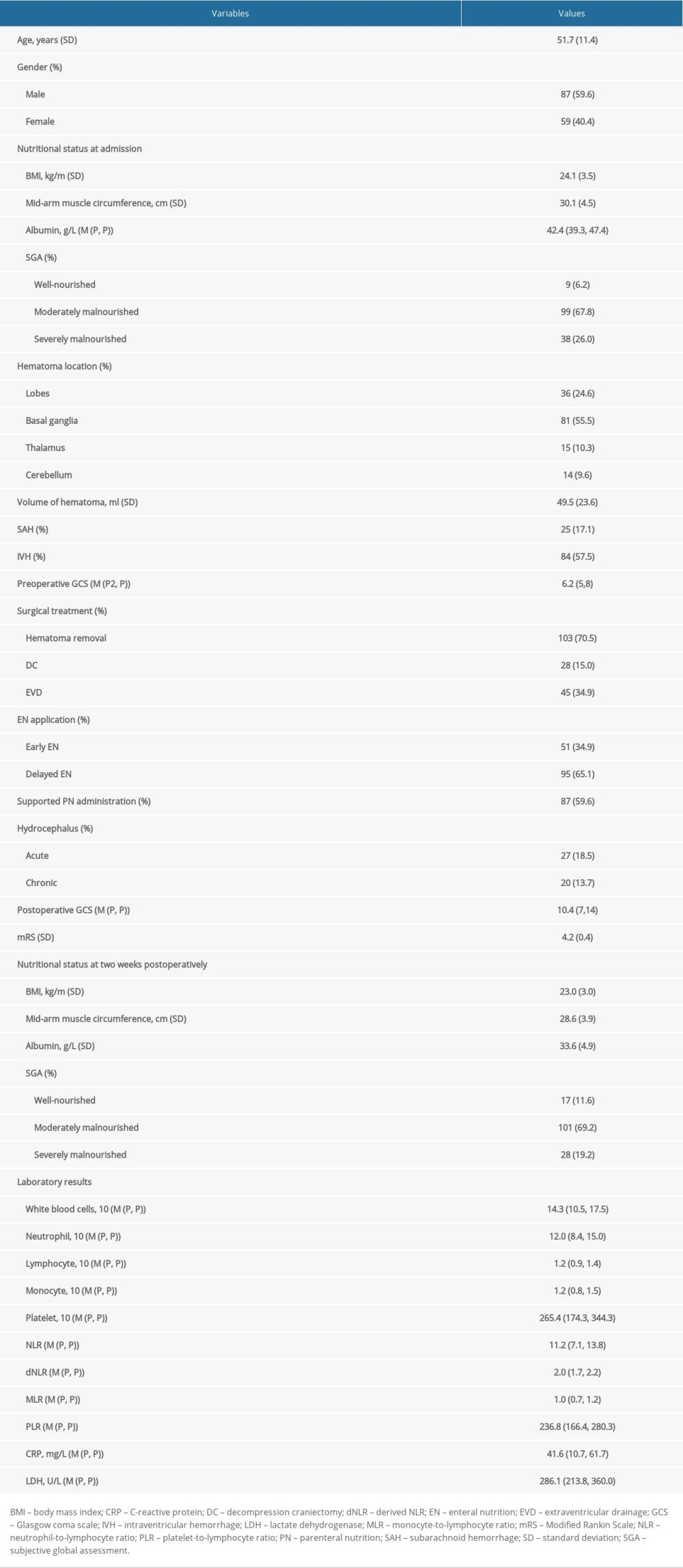 Table 2. The comparative analysis of patients with hydrocephalus.
Table 2. The comparative analysis of patients with hydrocephalus.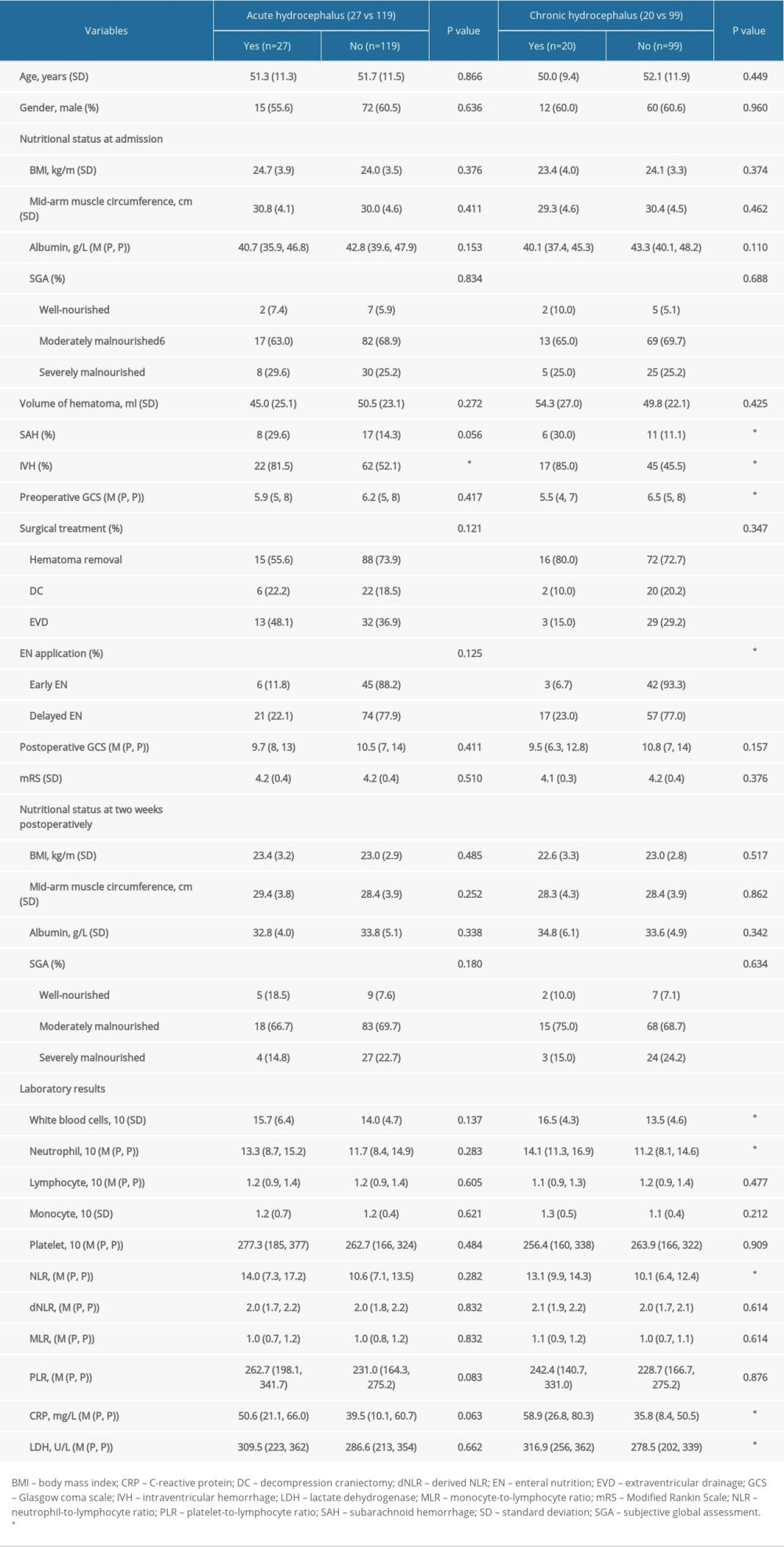 Table 3. Multivariate regression analysis of predictors for chronic hydrocephalus occurrence.
Table 3. Multivariate regression analysis of predictors for chronic hydrocephalus occurrence.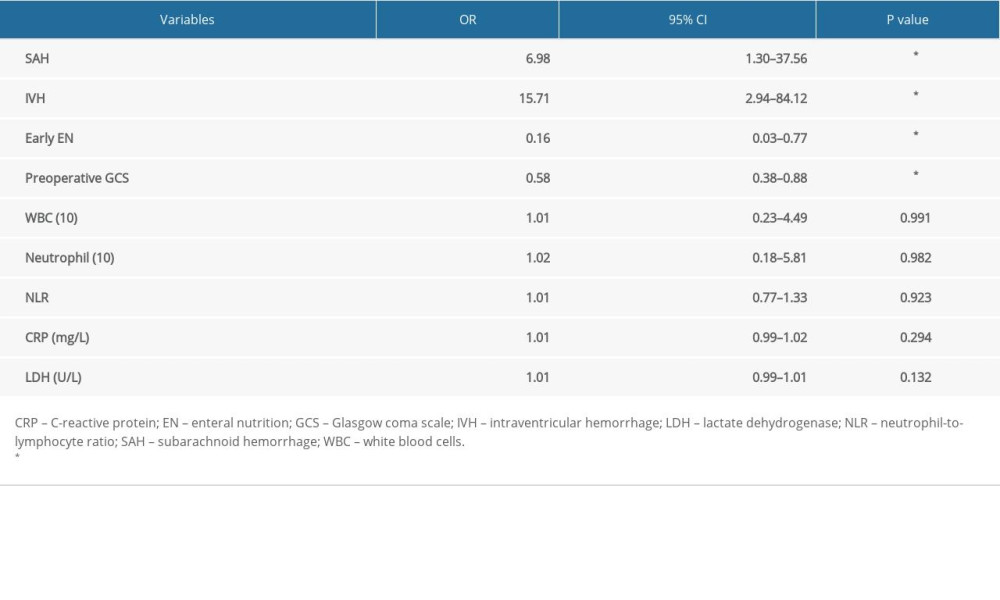 Table 4. The relationship between EN application and postoperative laboratory examinations of systematic inflammation reaction.
Table 4. The relationship between EN application and postoperative laboratory examinations of systematic inflammation reaction.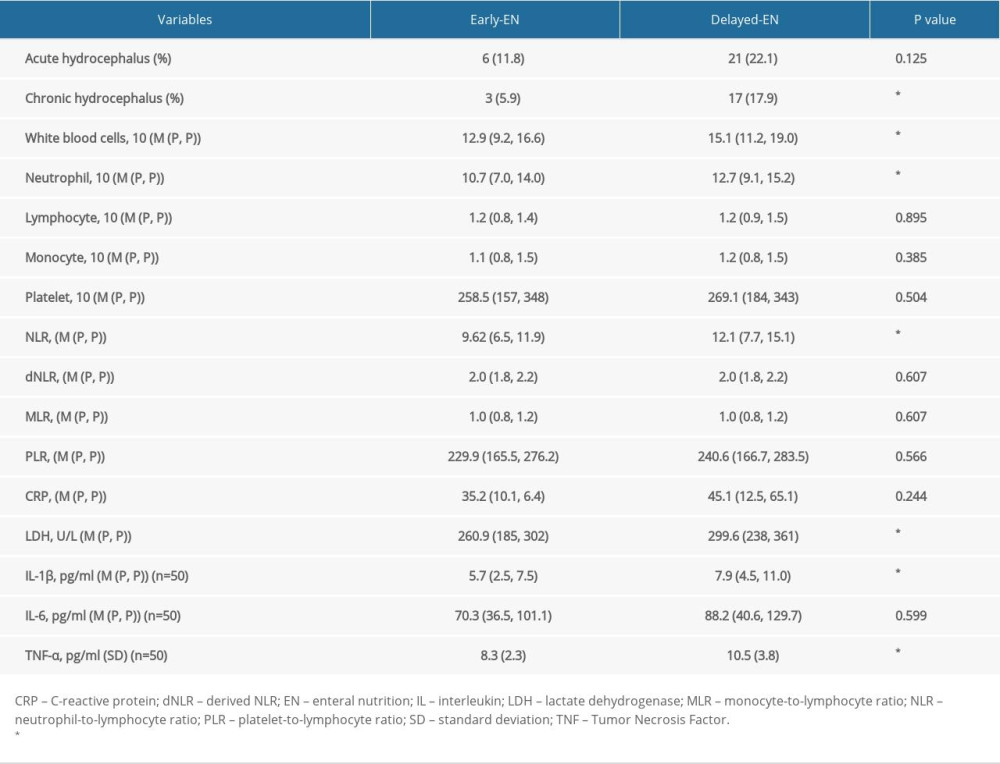
References
1. Meretoja A, Strbian D, Putaala J, SMASH-U: A proposal for etiologic classification of intracerebral hemorrhage: Stroke, 2012; 43(10); 2592-97
2. Gross BA, Jankowitz BT, Friedlander RM, Cerebral intraparenchymal hemorrhage: A review: JAMA, 2019; 321(13); 1295-303
3. Xu X, Chen X, Li F, Effectiveness of endoscopic surgery for supratentorial hypertensive intracerebral hemorrhage: A comparison with craniotomy: J Neurosurg, 2018; 128(2); 553-59
4. Veltkamp R, Purrucker J, Management of spontaneous intracerebral hemorrhage: Curr Neurol Neurosci Rep, 2017; 17(10); 80
5. Stein M, Luecke M, Preuss M, Spontaneous intracerebral hemorrhage with ventricular extension and the grading of obstructive hydrocephalus: The prediction of outcome of a special life-threatening entity: Neurosurgery, 2010; 67(5); 1243-51 discussion 1252
6. Giordan E, Palandri G, Lanzino G, Outcomes and complications of different surgical treatments for idiopathic normal pressure hydrocephalus: A systematic review and meta-analysis: J Neurosurg, 2018 [Online ahead of print]
7. Hu R, Zhang C, Xia J, Long-term outcomes and risk factors related to hydrocephalus after intracerebral hemorrhage: Transl Stroke Res, 2021; 12(1); 31-38
8. Karimy JK, Reeves BC, Damisah E, Inflammation in acquired hydrocephalus: pathogenic mechanisms and therapeutic targets: Nat Rev Neurol, 2020; 16(5); 285-96
9. Chen S, Yang Q, Chen G, Zhang JH, An update on inflammation in the acute phase of intracerebral hemorrhage: Transl Stroke Res, 2015; 6(1); 4-8
10. Kurland DB, Khaladj-Ghom A, Stokum JA, Complications associated with decompressive craniectomy: A systematic review: Neurocrit Care, 2015; 23(2); 292-304
11. Ki HJ, Lee HJ, Lee HJ, The risk factors for hydrocephalus and subdural hygroma after decompressive craniectomy in head injured patients: J Korean Neurosurg Soc, 2015; 58(3); 254-61
12. Chaudhry SR, Stoffel-Wagner B, Kinfe TM, Elevated systemic IL-6 levels in patients with aneurysmal subarachnoid hemorrhage is an unspecific marker for post-SAH complications: Int J Mol Sci, 2017; 18(12); 2580
13. Thwaites GE, Macmullen-Price J, Tran TH, Serial MRI to determine the effect of dexamethasone on the cerebral pathology of tuberculous meningitis: An observational study: Lancet Neurol, 2007; 6(3); 230-36
14. You S, Zheng D, Delcourt C, Determinants of early versus delayed neurological deterioration in intracerebral hemorrhage: Stroke, 2019; 50(6); 1409-14
15. Cheng X, Ru W, Du K, Association between enteral nutrition support and neurological outcome in patients with acute intracranial haemorrhage: A retrospective cohort study: Sci Rep, 2019; 9(1); 16507
16. Reintam Blaser A, Starkopf J, Alhazzani W, Early enteral nutrition in critically ill patients: ESICM clinical practice guidelines: Intensive Care Med, 2017; 43(3); 380-98
17. Apelgren KN, Rombeau JL, Twomey PL, Miller RA, Comparison of nutritional indices and outcome in critically ill patients: Crit Care Med, 1982; 10(5); 305-7
18. Sun JK, Zhang WH, Chen WX, Effects of early enteral nutrition on Th17/Treg cells and IL-23/IL-17 in septic patients: World J Gastroenterol, 2019; 25(22); 2799-808
19. Horwat P, Kopeć S, Garczyk A, Influence of enteral nutrition on gut microbiota composition in patients with Crohn’s disease: A systematic review: Nutrients, 2020; 12(9); 2551
20. Zong L, Li H, Li S, Effects of neoadjuvant chemotherapy combined with enteral nutrition on perioperative immunity, inflammation and intestinal flora in gastric cancer patients: J BOUN, 2019; 24(3); 1113-19
21. Bateman RM, Sharpe MD, Jagger JE: Crit Care, 2016; 20(Suppl 2); 94
22. Härtl R, Gerber LM, Ni Q, Ghajar J, Effect of early nutrition on deaths due to severe traumatic brain injury: J Neurosurg, 2008; 109(1); 50-56
23. Yang K, Feng Y, Mu J, The presence of previous cerebral microbleeds has a negative effect on hypertensive intracerebral hemorrhage recovery: Front Aging Neurosci, 2017; 9; 49
24. Li L, Molian VA, Seaman SC, Impact of intracerebral hematoma evacuation during decompressive hemicraniectomy on functional outcomes: Stroke, 2021; 52(3); 1105-8
25. Hemphill JC, Greenberg SM, Anderson CS, Guidelines for the management of spontaneous intracerebral hemorrhage: A guideline for healthcare professionals from the American Heart Association /American Stroke Association: Stroke, 2015; 46(7); 2032-60
26. Kondrup J, Allison SP, Elia M, ESPEN guidelines for nutrition screening 2002: Clin Nutr, 2003; 22(4); 415-21
27. Findlay M, White K, Brown C, Bauer JD, Nutritional status and skeletal muscle status in patients with head and neck cancer: Impact on outcomes: J Cachexia Sarcopenia Muscle, 2021; 12(6); 2187-98
28. Jain G, Mukerji G, Dixit A, The impact of nutritional status on the outcome of Indian patients undergoing neurosurgical shunt surgery: Br J Nutr, 2007; 98(5); 944-49
29. Harvey SE, Parrott F, Harrison DA, Trial of the route of early nutritional support in critically ill adults: N Engl J Med, 2014; 371(18); 1673-84
30. Morgenstern LB, Hemphill JC, Anderson C, Guidelines for the management of spontaneous intracerebral hemorrhage: A guideline for healthcare professionals from the American Heart Association/American Stroke Association: Stroke, 2010; 41(9); 2108-29
31. Hanley DF, Thompson RE, Rosenblum M, Efficacy and safety of minimally invasive surgery with thrombolysis in intracerebral haemorrhage evacuation (MISTIE III): A randomised, controlled, open-label, blinded endpoint phase 3 trial: Lancet, 2019; 393(10175); 1021-32
32. Toma AK, Holl E, Kitchen ND, Watkins LD, Evans’ index revisited: The need for an alternative in normal pressure hydrocephalus: Neurosurgery, 2011; 68(4); 939-44
33. Yang WS, Shen YQ, Zhang XD, Hydrocephalus growth: Definition, prevalence, association with poor outcome in acute intracerebral hemorrhage: Neurocrit Care, 2021; 35(1); 62-71
34. Hughes JD, Puffe R, Rabinstein AA, Risk factors for hydrocephalus requiring external ventricular drainage in patients with intraventricular hemorrhage: J Neurosurg, 2015; 123(6); 1439-46
35. Bhattathiri PS, Gregson B, Prasad KS, Mendelow AD, Intraventricular hemorrhage and hydrocephalus after spontaneous intracerebral hemorrhage: Results from the STICH trial: Acta Neurochir Suppl, 2006; 96; 65-68
36. Gopalakrishnan MS, Shanbhag NC, Shukla DP, Complications of decompressive craniectomy: Front Neurol, 2018; 9; 977
37. Tan X, Chen J, Keep RF, Xi G, Hua Y, Prx2 (Peroxiredoxin 2) as a cause of hydrocephalus after intraventricular hemorrhage: Stroke, 2020; 51(5); 1578-86
38. Czubowicz K, Głowacki M, Fersten E, Levels of selected pro- and anti-inflammatory cytokines in cerebrospinal fluid in patients with hydrocephalus: Folia Neuropathol, 2017; 55(4); 301-7
39. Schmidt MJ, Rummel C, Hauer J, Increased CSF aquaporin-4, and interleukin-6 levels in dogs with idiopathic communicating internal hydrocephalus and a decrease after ventriculo-peritoneal shunting: Fluids Barriers CNS, 2016; 13(1); 12
40. Karimy JK, Zhang J, Kurland DB, Inflammation-dependent cerebrospinal fluid hypersecretion by the choroid plexus epithelium in posthemorrhagic hydrocephalus: Nat Med, 2017; 23(8); 997-1003
41. Kanat A, Turkmenoglu O, Aydin MD, Toward changing of the pathophysiologic basis of acute hydrocephalus after subarachnoid hemorrhage: A preliminary experimental study: World Neurosurg, 2013; 80(3–4); 390-95
42. Park YK, Yi HJ, Choi KS, Predicting factors for shunt-dependent hydrocephalus in patients with aneurysmal subarachnoid hemorrhage: Acta Neurochir (Wien), 2018; 160(7); 1407-13
43. Carabotti M, Scirocco A, Maselli MA, Severi C, The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems: Ann Gastroenterol, 2015; 28(2); 203-9
44. Cryan JF, O’Riordan KJ, Sandhu K, The gut microbiome in neurological disorders: Lancet Neurol, 2020; 19(2); 179-94
45. Erny D, Hrabě de Angelis AL, Jaitin D, Host microbiota constantly control maturation and function of microglia in the CNS: Nat Neurosci, 2015; 18(7); 965-77
46. Tao G, Min-Hua C, Feng-Chan X, Changes of plasma acetylcholine and inflammatory markers in critically ill patients during early enteral nutrition: A prospective observational study: J Crit Care, 2019; 52; 219-26
47. Taylor BE, McClave SA, Martindale RG, Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.): Crit Care Med, 2016; 44(2); 390-438
48. Kudsk KA, Beneficial effect of enteral feeding: Gastrointest Endosc Clin N Am, 2007; 17(4); 647-62
49. Kudsk KA, Current aspects of mucosal immunology and its influence by nutrition: Am J Surg, 2002; 183(4); 390-98
50. Gurnari C, Breccia M, Di Giuliano F, Early intracranial haemorrhages in acute promyelocytic leukaemia: Analysis of neuroradiological and clinico-biological parameters: Br J Haematol, 2021; 193(1); 129-32
Figures
 Figure 1. The diagnosis of hydrocephalus was performed by using an Evan index method. The distance between the frontal horns of the lateral ventricles (full line indicated with ‘a’) and the longest inner diameter of skull at the same plane (dotted line indicated with ‘b’). The Evans index was calculated as the ratio of a to b. (Adobe Photoshop CC, 14.0, Adobe).
Figure 1. The diagnosis of hydrocephalus was performed by using an Evan index method. The distance between the frontal horns of the lateral ventricles (full line indicated with ‘a’) and the longest inner diameter of skull at the same plane (dotted line indicated with ‘b’). The Evans index was calculated as the ratio of a to b. (Adobe Photoshop CC, 14.0, Adobe). Figure 2. Representative CT images from HICH patients. The patients with severe HICH underwent microsurgery for hematoma removal followed decompression craniectomy received early EN (A, B) and delayed EN (C, D) treatment, respectively. CT images were acquired at admission (A, C) and at 3 months (B, D) postoperatively. Patient with early EN did not develop hydrocephalus, while patient with delayed EN developed chronic hydrocephalus during follow-up. (Adobe Photoshop CC, 14.0, Adobe).
Figure 2. Representative CT images from HICH patients. The patients with severe HICH underwent microsurgery for hematoma removal followed decompression craniectomy received early EN (A, B) and delayed EN (C, D) treatment, respectively. CT images were acquired at admission (A, C) and at 3 months (B, D) postoperatively. Patient with early EN did not develop hydrocephalus, while patient with delayed EN developed chronic hydrocephalus during follow-up. (Adobe Photoshop CC, 14.0, Adobe). Tables
 Table 1. The clinical characteristics of 146 patients with severe HICH.
Table 1. The clinical characteristics of 146 patients with severe HICH. Table 2. The comparative analysis of patients with hydrocephalus.
Table 2. The comparative analysis of patients with hydrocephalus. Table 3. Multivariate regression analysis of predictors for chronic hydrocephalus occurrence.
Table 3. Multivariate regression analysis of predictors for chronic hydrocephalus occurrence. Table 4. The relationship between EN application and postoperative laboratory examinations of systematic inflammation reaction.
Table 4. The relationship between EN application and postoperative laboratory examinations of systematic inflammation reaction. Table 1. The clinical characteristics of 146 patients with severe HICH.
Table 1. The clinical characteristics of 146 patients with severe HICH. Table 2. The comparative analysis of patients with hydrocephalus.
Table 2. The comparative analysis of patients with hydrocephalus. Table 3. Multivariate regression analysis of predictors for chronic hydrocephalus occurrence.
Table 3. Multivariate regression analysis of predictors for chronic hydrocephalus occurrence. Table 4. The relationship between EN application and postoperative laboratory examinations of systematic inflammation reaction.
Table 4. The relationship between EN application and postoperative laboratory examinations of systematic inflammation reaction. In Press
05 Mar 2024 : Clinical Research
Role of Critical Shoulder Angle in Degenerative Type Rotator Cuff Tears: A Turkish Cohort StudyMed Sci Monit In Press; DOI: 10.12659/MSM.943703
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








