20 June 2022: Clinical Research
A Single-Center Retrospective Study to Compare the Efficacy and Safety of Modified FOLFIRINOX with S-1 as Adjuvant Chemotherapy in 71 Patients with Resected Pancreatic Carcinoma
Linhua Yao1ABCEF, Chengwu Tang2ABCEF, Wenming Feng2ABFG, Hanbin Dai2ABCE*DOI: 10.12659/MSM.937136
Med Sci Monit 2022; 28:e937136
Abstract
BACKGROUND: Studies are ongoing to determine the optimal adjuvant chemotherapy (ACT) for resected pancreatic carcinoma (PC). FOLFIRINOX is a chemotherapy regimen including oxaliplatin, irinotecan, leucovorin, and 5-fluorouracil (5-FU). S-1 is a fluoropyrimidine derivative widely used as ACT for gastrointestinal malignancy. This single-center retrospective study aimed to compare the efficacy and safety of modified FOLFIRINOX (mFOLFIRINOX) with S-1 as ACT for resected PC.
MATERIAL AND METHODS: A total of 71 patients with PC who accepted ACT after R0 resection between February 2016 and January 2019 were enrolled in this retrospective study. Among these patients, 34 received mFOLFIRINOX regimen chemotherapy (mFFX group), while 37 received S-1 monochemotherapy (S-1 group). The mFOLFIRINOX regimen included oxaliplatin 65 mg/m², leucovorin 400 mg/m², irinotecan 150 mg/m², 5-FU 400 mg/m², and continuous 5-FU 2400 mg/m² (for 46 h), in a 2-week schedule. The S-1 monochemotherapy (80-120 mg/day according to body surface area [BSA], in 2 divided doses for 2 week) was administrated every 3 weeks. We followed up these patients and analyzed the relapse-free survival (RFS), overall survival (OS), and chemotherapy-induced adverse events (AEs).
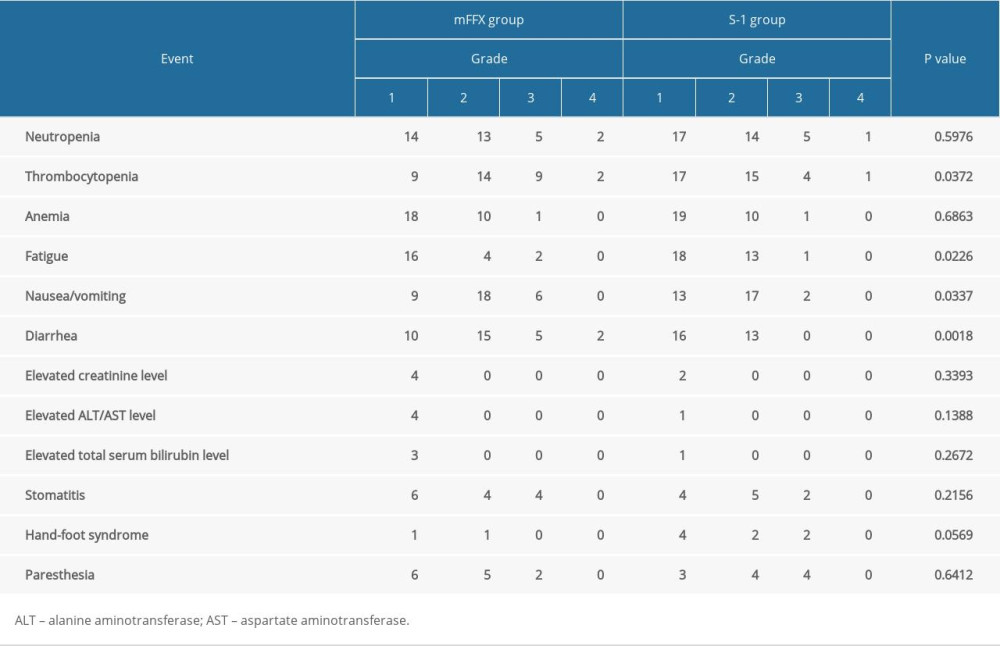
RESULTS: The mFFX group demonstrated a markedly higher 3-year RFS (P=0.0332) and OS (P=0.0346) than the S-1 group. Patients in the mFFX group experienced significantly more common and severe thrombocytopenia (P=0.0372), fatigue (P=0.0226), nausea/vomiting (P=0.0337), and diarrhea (P=0.0018). No chemotherapy-induced death was documented.
CONCLUSIONS: This retrospective study indicated that if dose adjustment and adverse events management are properly administrated, mFOLFIRINOX regimen chemotherapy could result in an improved survival compared with S-1 monochemotherapy for resected PC.
Keywords: Chemotherapy, Adjuvant, pancreatic carcinoma, Recurrence, Survival, Antineoplastic Combined Chemotherapy Protocols, Fluorouracil, Humans, irinotecan, leucovorin, Neoplasm Recurrence, Local, oxaliplatin, Pancreatic Neoplasms
Background
Pancreatic carcinoma (PC) causes 466 000 deaths yearly because of its dismal prognosis and is the seventh most lethal malignancy globally [1]. Despite advances in diagnostic and management of PC, both incidence and mortality rates of PC have gradually risen through the last few decades [2]. Radical surgery provides the only opportunity for cure, whereas over 90% of the patients relapse postoperatively [3].
Adjuvant chemotherapy (ACT) has been proven to effectively improve prognosis and is recommended as a necessary postoperative treatment for PC [4]. Gemcitabine and gemcitabine-based regimens are used as first-line regimens of ACT and lead to remarkable survival improvement among patients with resected PC [5,6]. Recently, regimens with promising clinical efficacy were reported. The JASPAC-01 trial revealed the superiority of S-1 over gemcitabine in the Asian population [7].
The FOLFIRINOX regimen including oxaliplatin, irinotecan, leucovorin, and 5-fluorouracil (5-FU) was found to achieve longer overall survival (OS) than gemcitabine in patients with metastatic PC [8]. More recently, the PRODIGE 24-ACCORD 24-CCTG PA 6 trial presented the survival benefits of the modified FOLFIRINOX (mFOLFIRINOX) regimen relative to gemcitabine, both in relapse-free survival (RFS) and OS [9].
Therefore, this single-center retrospective study aimed to compare the efficacy and safety of mFOLFIRINOX with S-1 as ACT for patients with resected PC.
Material and Methods
PATIENTS:
This study was conducted following the Helsinki Declaration principles and Good Clinical Practice recommendations and was approved by the Medical Research Ethics Committee of First People’s Hospital affiliated to Huzhou Normal College. Patient informed consent was waived.
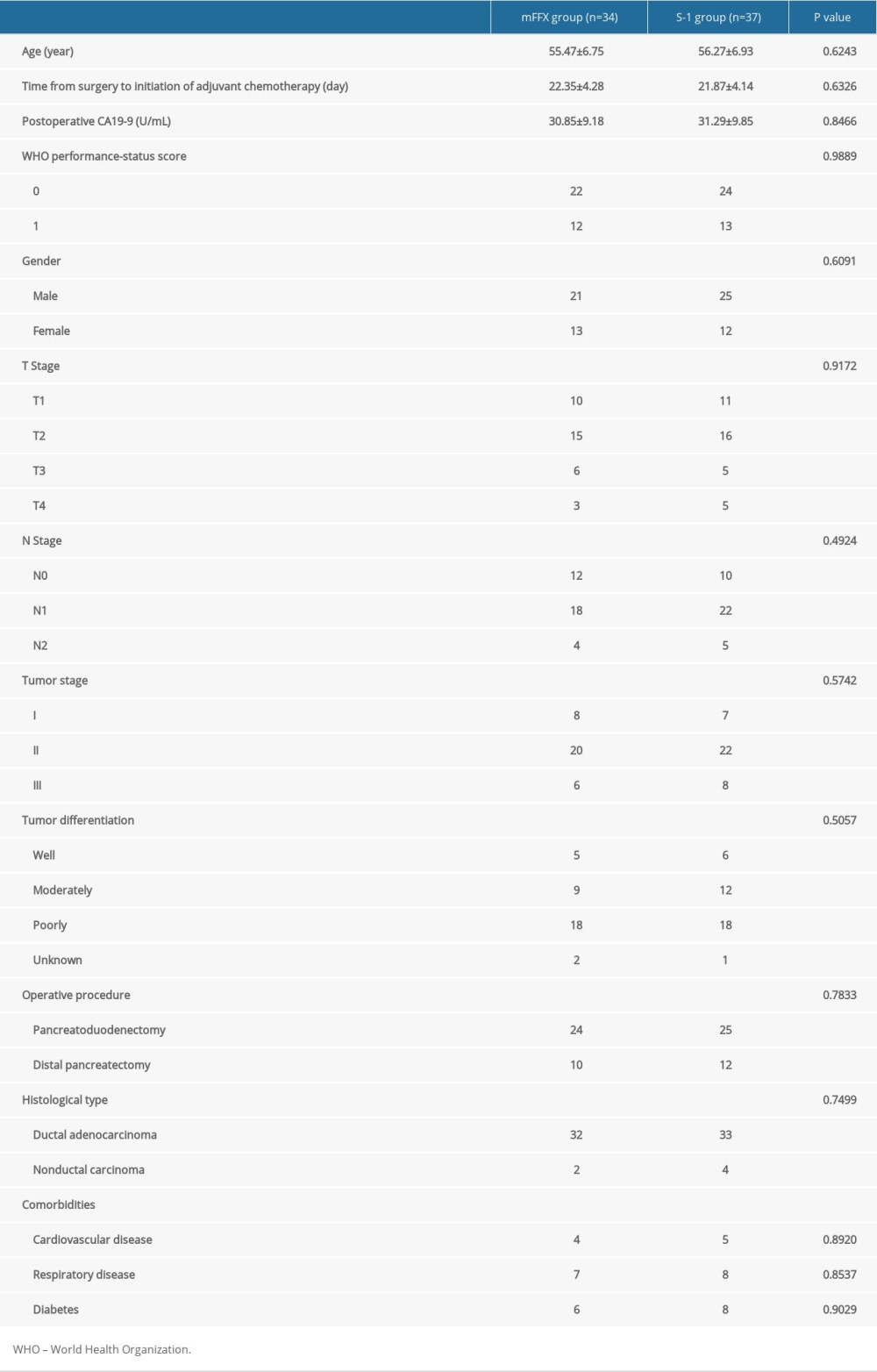
We enrolled 71 patients with PC who underwent ACT after R0 resection between February 2016 and January 2019. Eligibility criteria were: ages 18–75 years; histologically confirmed pancreatic adenocarcinoma; no neoadjuvant chemotherapy; World Health Organization (WHO) performance-status score of 0 or 1; and sufficient organ function. Patients were not eligible if they were lost to follow-up or had a history of other malignancies. Among these patients, 34 received mFOLFIRINOX regimen chemotherapy (mFFX group), while 37 received S-1 monochemotherapy (S-1 group).
CHEMOTHERAPY ADMINISTRATION:
Adjuvant chemotherapy was initiated within 4 weeks postoperatively. Patients were fully informed before choosing the chemotherapy regimen. The mFOLFIRINOX regimen included oxaliplatin 65 mg/m2, leucovorin 400mg/m2, irinotecan 150 mg/m2, 5-FU 400 mg/m2, and continuous 5-FU 2400 m42 (for 46 h), in a 2-week schedule. The dose of the mFOLFIRINOX regimen was modified according to physical condition of the Chinese population and previous regimens used in PC4[10]. The S-1 monochemotherapy (body surface area [BSA] ≥1.5 m2, 120 mg/day; 1.25 m2 ≤BSA <1.5 m2, 100 mg/day; BSA <1.25 m2, 80 mg/day, in 2 divided doses for 2 weeks) was administrated every 3 weeks.
Chemotherapy-induced adverse events (AEs) were graded based on the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0. A 25% dose reduction was applied subsequently in the event of grade 2 or 3 hand–foot syndrome, grade 4 neutropenia, grade 3 or 4 thrombocytopenia or anemia, or another grade 3 or 4 acute non-hematologic AEs. ACT was terminated in case of overwhelming chemotherapy-induced AEs, relapse, or the patients’ request for discontinuation. Patients accepting fewer than 4 cycles of ACT were not eligible for analysis.
PATIENT ASSESSMENT AND FOLLOW-UP:
All patients received routine health assessment and disease monitoring at the start of each treatment cycle. We followed up these patients monthly during the first postoperative year, and then quarterly until the last follow-up or death. Relapse was confirmed based on imaging and histological examination. Once relapse was diagnosed, chemotherapy of alternative regimen, radiofrequency ablation, or palliative treatment was implemented.
STATISTICAL ANALYSIS:
MedCalc software (version 15.2.2, MedCalc Software, Ltd) was used for statistical analysis and visualization. Quantitative data were shown as mean±SD and compared using the
Results
PATIENT CHARACTERISTICS:
Patient characteristics, including sex, comorbidities, operative procedure, postoperative CA19-9 level, age, tumor differentiation, histological type, TNM stage (on the basis of the 8th edition of AJCC/UICC staging system) [11], and time from surgery to initiation of ACT did not differ significantly between the 2 groups (Table 1).
CHEMOTHERAPY-INDUCED ADVERSE EVENTS AND TREATMENT OUTCOMES:
The severity and incidence of chemotherapy-induced AEs are summarized in Table 2. Patients in the mFFX group experienced significantly more common and severe thrombocytopenia (P=0.0372), fatigue (P=0.0226), nausea/vomiting (P=0.0337), and diarrhea (P=0.0018). Most of the AEs were properly managed by symptomatic therapy and dose adjustment. No chemotherapy-induced death was documented.
The incidence of dose reduction was markedly greater in the mFFX group (14/34 vs 6/37,
RELAPSE-FREE SURVIVAL:
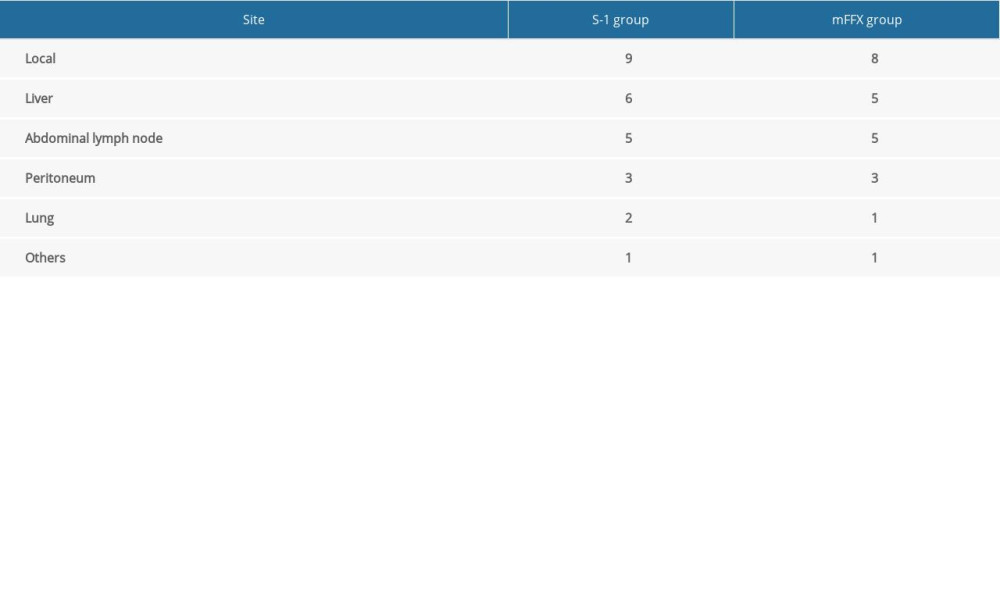
Relapse was confirmed in 23 patients of the mFFX group and 26 patients of the S-1 group, respectively, within 3 years postoperatively. The mFFX group demonstrated a markedly higher 3-year RFS (P=0.0332) and lower hazard ratio (HR) for relapse (0.5577, 95% confidence interval [CI], 0.3138 to 0.9910) than the S-1 group (Figure 1). Sites of first relapse are shown in Table 3.
OVERALL SURVIVAL:
A total of 46 patients died within 3 years postoperatively (22 from the mFFX group and 24 from the S-1 group). Compared with the S-1 group, the mFFX group obtained a significantly improved 3-year OS (P=0.0346) and lower HR for death (0.5501, 95% CI 0.3029 to 0.9993) (Figure 2).
Discussion
In this study, we retrospectively analyzed 71 patients with resected pancreatic carcinoma, among whom 34 received the mFOLFIRINOX regimen chemotherapy and 37 received S-1 monochemotherapy. The results indicated that the mFOLFIRINOX regimen resulted in a markedly improved 3-year RFS and lower HR for relapse compared to the S-1 group in patients with resected PC. The mFOLFIRINOX regimen achieved a significantly improved 3-year overall survival with a lower HR for death.
Pancreatic carcinoma remains a major medical dilemma, with an extremely poor prognosis worldwide [12]. Surgical resection improves survival outcomes. However, 80–85% of the patients were diagnosed at an advanced inoperable stage [13]. Despite the development of surgical techniques, relapse often occurs within 2 years after surgery, most of which are local recurrence and hepatic metastases [14]. Therefore, 5-year survival probabilities after surgical resection alone are unsatisfactory (under 10%) [15].
Therefore, postoperative adjuvant treatment modalities were explored. Survival benefits of 5-FU-based adjuvant chemotherapy vs adjuvant chemoradiotherapy were confirmed by the ESPAC-1 trial [16,17]. Subsequently, gemcitabine became a pivotal chemotherapeutic agent for PC, showing comparable survival benefits and fewer AEs according to the CONKO-001 trial and ESPAC-3 (version 2) trial [5,18].
S-1 is an innovative oral chemotherapeutic agent used for resected gastric cancer in Asian countries [19]. According to recent studies, S-1 conferred a higher response and improved overall survival than gemcitabine in metastatic and resected PC [7,20,21]. Therefore, S-1 monochemotherapy was recognized as a new standard adjuvant chemotherapy regimen for PC. The mFOLFIRINOX regimen is another competitive chemotherapy regimen with superior long-term survival to gemcitabine for resected PC. The MPACA-3 trial compared the efficacy of mFOLFIRINOX with S-1 as second-line chemotherapy in metastatic PC patients who were failed after gemcitabine chemotherapy, and demonstrated an increased overall survival compared to S-1 [22]. However, the efficacy of these 2 regimens as first-line chemotherapy in resected PC has not been compared. So far as we know, this single-center retrospective study was the first to directly compare the efficacy of mFOLFIRINOX with S-1 as ACT for patients with resected PC.
The safety of these 2 regimens was also compared. Patients who accepted mFOLFIRINOX regimen chemotherapy experienced significantly more common and severe thrombocytopenia, fatigue, nausea/vomiting, and diarrhea. Therefore, the incidence of dose reduction was significantly greater in the mFFX group. Eventually, most of the patients finished treatment, and no chemotherapy-induced death occurred.
The limitations of this study are non-negligible. As a retrospective cohort study, bias in patient enrollment was unavoidable. Due to the scale of our hospital, the sample size was relatively limited. Hence, we are considering performing a multicentre, prospective, randomized trial.
Conclusions
This retrospective study indicates that if dose adjustment and adverse events management are properly administered, the mFOLFIRINOX regimen chemotherapy can result in better survival than S-1 monochemotherapy for patients with resected PC.
Figures
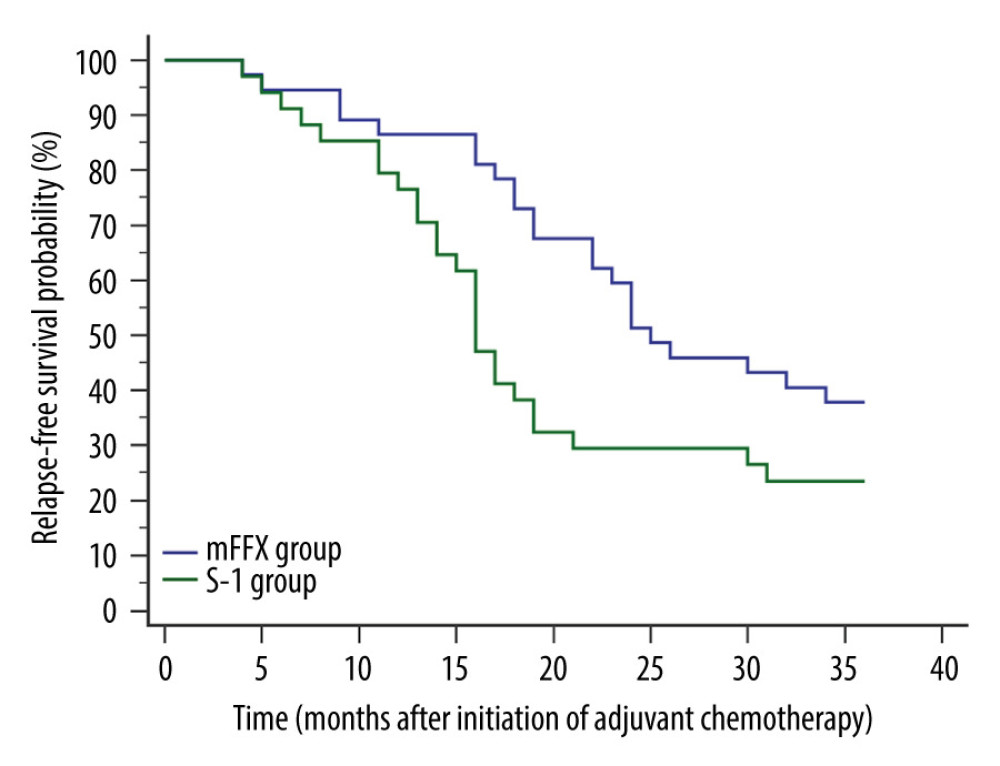 Figure 1. Comparison of relapse-free survival. Relapse was confirmed in 23 patients of the mFFX group and 26 patients of the S-1 group within 3 years postoperatively. The mFFX group demonstrated a markedly higher 3-year relapse-free survival (P=0.0332) and lower hazard ratio for relapse (0.5577, 95% CI, 0.3138 to 0.9910) than the S-1 group. MedCalc software (version 15.2.2, MedCalc Software, Ltd.) was used to create the figure.
Figure 1. Comparison of relapse-free survival. Relapse was confirmed in 23 patients of the mFFX group and 26 patients of the S-1 group within 3 years postoperatively. The mFFX group demonstrated a markedly higher 3-year relapse-free survival (P=0.0332) and lower hazard ratio for relapse (0.5577, 95% CI, 0.3138 to 0.9910) than the S-1 group. MedCalc software (version 15.2.2, MedCalc Software, Ltd.) was used to create the figure. 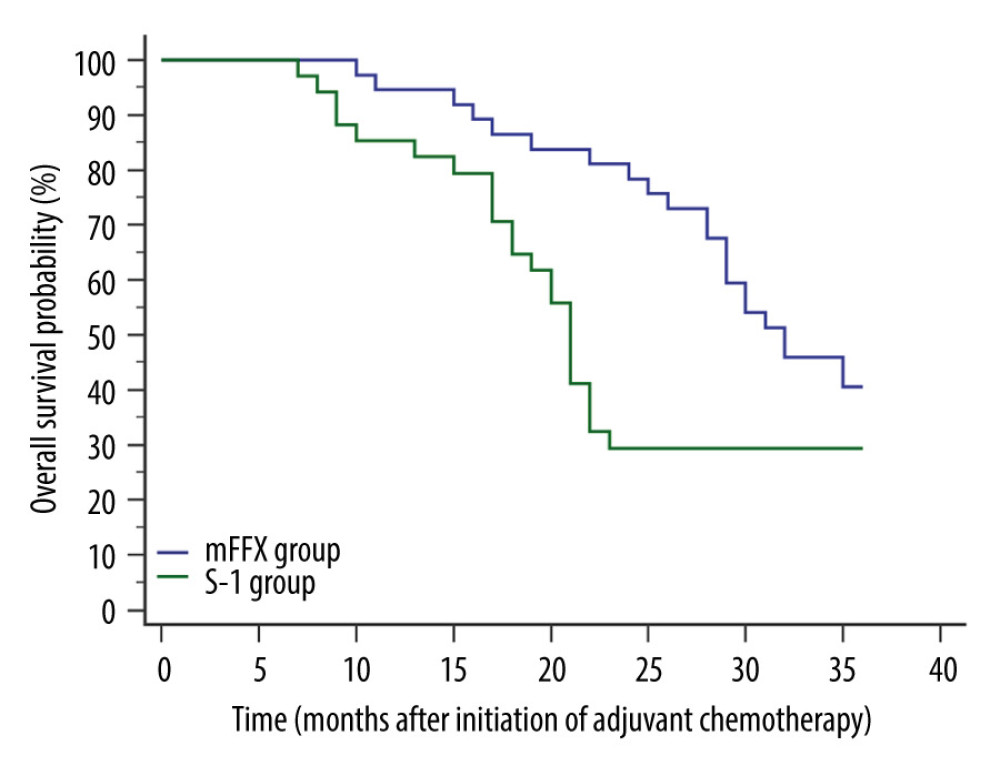 Figure 2. Comparison of overall survival. A total of 46 patients died within 3 years postoperatively (22 from the mFFX group and 24 from the S-1 group). Compared with the S-1 group, the mFFX group obtained a significantly improved 3-year overall survival (P=0.0346) and lower hazard ratio for death (0.5501, 95% CI 0.3029 to 0.9993). MedCalc software (version 15.2.2, MedCalc Software, Ltd.) was used to create the figure.
Figure 2. Comparison of overall survival. A total of 46 patients died within 3 years postoperatively (22 from the mFFX group and 24 from the S-1 group). Compared with the S-1 group, the mFFX group obtained a significantly improved 3-year overall survival (P=0.0346) and lower hazard ratio for death (0.5501, 95% CI 0.3029 to 0.9993). MedCalc software (version 15.2.2, MedCalc Software, Ltd.) was used to create the figure. References
1. Sung H, Ferlay J, Siegel RL, Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries: Cancer J Clin, 2021; 71(3); 209-49
2. Ryan DP, Hong TS, Bardeesy N, Pancreatic adenocarcinoma: N Engl J Med, 2014; 371(11); 1039-49
3. Sinn M, Bahra M, Liersch T, CONKO-005: Adjuvant chemotherapy with gemcitabine plus erlotinib versus gemcitabine alone in patients after R0 resection of pancreatic cancer: A multicenter randomized phase III trial: J Clin Oncol, 2017; 35(29); 3330-37
4. Khorana AA, McKernin SE, Berlin J, Potentially curable pancreatic adenocarcinoma: ASCO clinical practice guideline update: J Clin Oncol, 2019; 37(23); 2082-88
5. Oettle H, Neuhaus P, Hochhaus A, Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: The CONKO-001 randomized trial: JAMA, 2013; 310(14); 1473-81
6. Oettle H, Post S, Neuhaus P, Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: A randomized controlled trial: Jama, 2007; 297(3); 267-77
7. Uesaka K, Boku N, Fukutomi A, Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: A phase 3, open-label, randomised, non-inferiority trial (JASPAC 01): Lancet, 2016; 388(10041); 248-57
8. Conroy T, Desseigne F, Ychou M, FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer: N Engl J Med, 2011; 364(19); 1817-25
9. Conroy T, Hammel P, Hebbar M, FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer: N Engl J Med, 2018; 379(25); 2395-406
10. Zou L, Li X, Wu X, Modified FOLFIRINOX versus gemcitabine plus oxaliplatin as first-line chemotherapy for patients with locally advanced or metastatic cholangiocarcinoma: A retrospective comparative study: BMC Cancer, 2021; 21(1); 818
11. Chun YS, Pawlik TM, Vauthey JN: Ann Surg Oncol, 2018; 25(4); 845-47
12. Vincent A, Herman J, Schulick R, Pancreatic cancer: Lancet, 2011; 378(9791); 607-20
13. Ansari D, Tingstedt B, Andersson B, Pancreatic cancer: Yesterday, today and tomorrow: Future Oncol, 2016; 12(16); 1929-46
14. Sperti C, Pasquali C, Piccoli A, Pedrazzoli S, Recurrence after resection for ductal adenocarcinoma of the pancreas: World J Surg, 1997; 21(2); 195-200
15. Fenocchio E, Filippi R, Lombardi P, Is there a standard adjuvant therapy for resected pancreatic cancer?: Cancers, 2019; 11(10); 1547
16. Neoptolemos JP, Dunn JA, Stocken DD, Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: A randomised controlled trial: Lancet, 2001; 358(9293); 1576-85
17. Neoptolemos JP, Stocken DD, Friess H, A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer: N Engl J Med, 2004; 350(12); 1200-10
18. Neoptolemos JP, Stocken DD, Bassi C, Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: A randomized controlled trial: JAMA, 2010; 304(10); 1073-81
19. Sakuramoto S, Sasako M, Yamaguchi T, Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine: N Engl J Med, 2007; 357(18); 1810-20
20. Ueno H, Okusaka T, Ikeda M, An early phase II study of S-1 in patients with metastatic pancreatic cancer: Oncology, 2005; 68(2–3); 171-78
21. Okusaka T, Funakoshi A, Furuse J, A late phase II study of S-1 for metastatic pancreatic cancer: Cancer Chemother Pharmacol, 2008; 61(4); 615-21
22. Go SI, Lee SC, Bae WK, Modified FOLFIRINOX versus S-1 as second-line chemotherapy in gemcitabine-failed metastatic pancreatic cancer patients: A randomised controlled trial (MPACA-3): Eur J Cancer, 2021; 157; 21-30
Figures
 Figure 1. Comparison of relapse-free survival. Relapse was confirmed in 23 patients of the mFFX group and 26 patients of the S-1 group within 3 years postoperatively. The mFFX group demonstrated a markedly higher 3-year relapse-free survival (P=0.0332) and lower hazard ratio for relapse (0.5577, 95% CI, 0.3138 to 0.9910) than the S-1 group. MedCalc software (version 15.2.2, MedCalc Software, Ltd.) was used to create the figure.
Figure 1. Comparison of relapse-free survival. Relapse was confirmed in 23 patients of the mFFX group and 26 patients of the S-1 group within 3 years postoperatively. The mFFX group demonstrated a markedly higher 3-year relapse-free survival (P=0.0332) and lower hazard ratio for relapse (0.5577, 95% CI, 0.3138 to 0.9910) than the S-1 group. MedCalc software (version 15.2.2, MedCalc Software, Ltd.) was used to create the figure. Figure 2. Comparison of overall survival. A total of 46 patients died within 3 years postoperatively (22 from the mFFX group and 24 from the S-1 group). Compared with the S-1 group, the mFFX group obtained a significantly improved 3-year overall survival (P=0.0346) and lower hazard ratio for death (0.5501, 95% CI 0.3029 to 0.9993). MedCalc software (version 15.2.2, MedCalc Software, Ltd.) was used to create the figure.
Figure 2. Comparison of overall survival. A total of 46 patients died within 3 years postoperatively (22 from the mFFX group and 24 from the S-1 group). Compared with the S-1 group, the mFFX group obtained a significantly improved 3-year overall survival (P=0.0346) and lower hazard ratio for death (0.5501, 95% CI 0.3029 to 0.9993). MedCalc software (version 15.2.2, MedCalc Software, Ltd.) was used to create the figure. In Press
05 Mar 2024 : Clinical Research
Role of Critical Shoulder Angle in Degenerative Type Rotator Cuff Tears: A Turkish Cohort StudyMed Sci Monit In Press; DOI: 10.12659/MSM.943703
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952