21 October 2022: Clinical Research
Distribution and Determinants of Plasma Homocysteine Levels in a Preconception Population: A Retrospective Single-Center Study
Manyu Wu1BCEF, Xinyan Huang1BC, Dan Liu1ADEF*DOI: 10.12659/MSM.937987
Med Sci Monit 2022; 28:e937987
Abstract
BACKGROUND: Raised plasma homocysteine (Hcy) levels have been associated with various diseases and pregnancy complications. Preconception is the primary prevention period to prevent birth defects. This retrospective study aimed to investigate the distribution of plasma Hcy levels among men and women at preconception and further evaluate the factors influencing plasma Hcy levels in a Southern China population.
MATERIAL AND METHODS: Sex, age, serum folate levels, plasma Hcy levels, and the time of Hcy and folate detection were obtained by medical records. Univariate analysis and multi-factor mixed virtual linear regression were used to explore the distribution and determinants of plasma Hcy levels.
RESULTS: A total of 3031 participants (1091 men [35.99%] and 1940 women [64.01%]) were included. The average levels of Hcy and the rates of hyperhomocysteinemia (HHcy) in men were higher than those in women (P<0.05). Hcy levels were observed to be lowest during autumn and highest during winter (P<0.05). In the normal Hcy (NHcy) group, serum folate levels were higher than in the HHcy group (P<0.05). Regression analysis suggested that sex, season, and serum folate levels had an effect on Hcy levels, but age was not an influencing factor of Hcy level in the preconception population.
CONCLUSIONS: This retrospective study showed that Hcy levels are higher in men and in the winter season. Sex, season, and serum folate levels were the influencing factors of Hcy in the preconception population.
Keywords: Folic Acid, homocysteine, Hyperhomocysteinemia, Preconception Care, Seasons, Humans, Male, Female, China
Background
The amino acid homocysteine (Hcy), which contains sulfur, is generally produced during methionine metabolism [1–3]. Several cross-sectional and prospective reports have presented the relationship between elevated plasma Hcy levels and more than 100 syndromes, diseases, and consequences, including ischemic stroke, bipolar disorder, cognitive decline, and cardiovascular disease [4–8]. Hyperhomocysteinemia (HHcy) is the term used to describe a condition when the Hcy level is >10 μmol/L [4,9,10]. Every 5 μmol/L rise in Hcy levels resulted in a 33.6% increase in mortality risk [11].
Since pregnancy is a very complicated physiological process, for the pregnancy to become successful, the uterus and the embryo need to communicate with each other properly. Any adverse change occurring in a pregnant woman’s physical state can complicate her pregnancy and lead to many adverse issues. Various studies have postulated that maternal Hcy-mediated placental thrombosis, inadequate placentation, apoptosis, inflammation, increased oxidative stress, and epigenetic alterations can lead to several pregnancy-linked issues [12–17], such as preeclampsia, placental abruption, early pregnancy loss, intrauterine growth restriction, and fetal death [18–20]. Normal Hcy values during pregnancy are 3.9 to 7.3 mmol/L before 16 gestational weeks, 3.5 to 5.3 mmol/L during gestational weeks 20 to 24, and 3.3 to 7.5 mmol/L after gestational week 36 [21]. Preconception care is provided for couples of reproductive age who are planning a pregnancy. Its aims are to identify pre-existing medical and social conditions that can affect pregnancy and the well-being of the mother and child. Preconception care also includes counseling for the parents.
Unhealthy physical conditions of the parents during the preconception period are linked to higher risks of morbidity and mortality in the child. Preconception care is advised by the World Health Organization as a means of enhancing the health and well-being of women and couples and improving the outcomes of subsequent pregnancies and child health [22].
A nationwide survey of residents ≥40 years of age from 31 provinces in the mainland of China showed that 25.9% of participants were defined as having Hhcy [23]. However, there are very few studies focused on the Hcy status of couples at preconception. The goal of this study was to investigate the distribution of plasma Hcy levels in men and women at preconception and further evaluate the vital variables that impact Hcy levels. Results of this study will provide significant new information about the Hcy status among couples at preconception and provide a preliminary theoretical basis for the future study on Hcy and adverse pregnancy outcomes and complications.
Material and Methods
PARTICIPANTS:
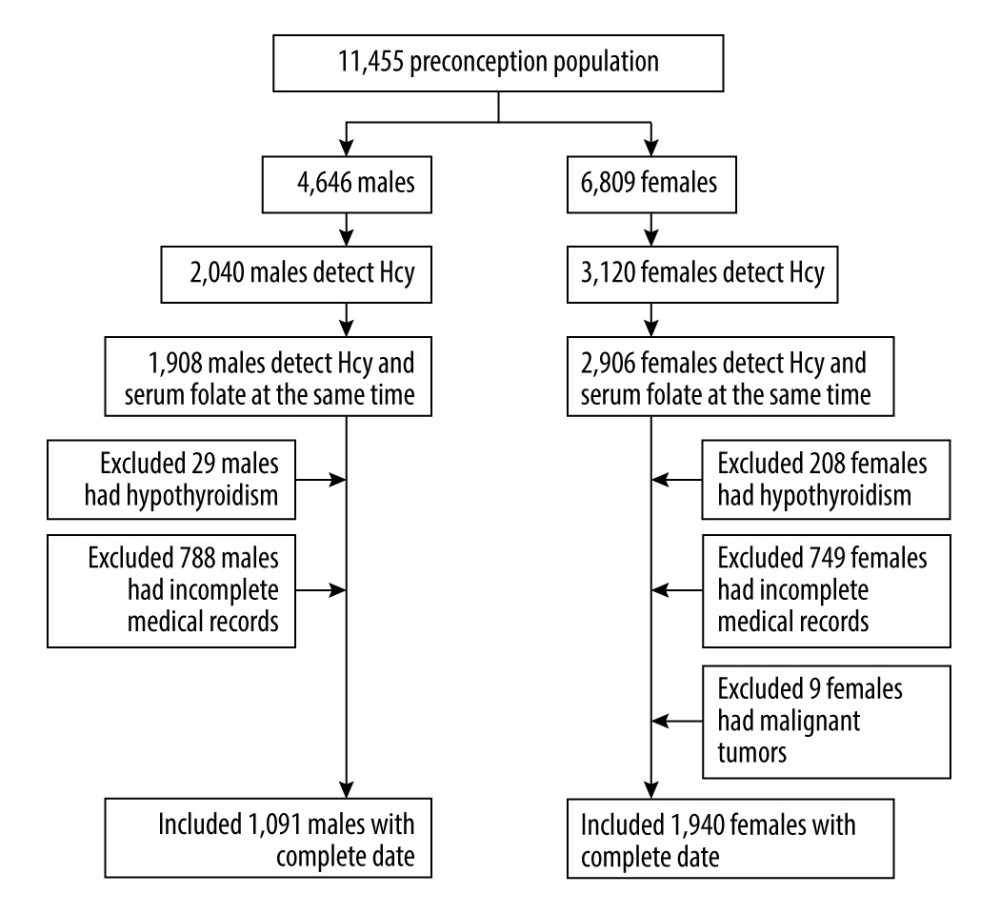
This retrospective study included couples who were seeking preconception care from January 2021 to May 2022 in the Department of Women’s Health Care, Affiliated Foshan Maternal and Child Health Hospital, Southern Medical University, a large preconception care center. Hcy levels and serum folate levels were tested at the first visit. People who had chronic renal failure, hypothyroidism, malignant tumors, or incomplete medical records or who had Hcy and serum folate levels that were not measured at the same time were excluded from this study. Ethical approval was waived by the Ethics Committee of Affiliated Foshan Women and Children Hospital, Southern Medical University in view of the retrospective nature of the study and because all performed procedures were part of routine care. All data were extracted from medical records, and the consent to participate was unavailable due to the retrospective design of the study and difficulty in reconnection with participants.
SAMPLE MEASUREMENT AND COLLECTION:
The Hcy and serum folate levels of the couples at preconception that were determined during their regular preconception care in the clinical laboratory were included in this report. After the participants fasted for 8 h, the nurses collected blood samples and immediately sent the samples for biochemical analyses in the hospital’s laboratory. An automated biochemical analyzer (AU5800, Beckman CoulterInc, Brea, CA, USA) was used for measuring Hcy levels, whereas an electrochemical luminescence automatic immune analyzer (Cobas e601, Roche Inc, Switzerland) was used for measuring serum folate levels. The time of measurement was also reviewed.
Hcy levels <10 μmol/L were defined as normal Hcy (NHcy), whereas Hcy levels ≥10 μmol/L were defined as HHcy. According to the meteorological classification, the winter season includes the months of December, January, and February; spring season includes the months of March, April, and May; summer season includes the months of June, July, and August; and autumn season includes the months of September, October, and November. Data related to relevant factors, such as serum folate levels, sex, age, Hcy levels, and time of folate and Hcy detection, were obtained after examining the medical records of the participants.
STATISTICAL ANALYSIS:
Statistical analyses were conducted with SPSS Windows software (version 20.0; IBM Corp, Armonk, NY, USA). All data were tested for normality. The data that showed a normal distribution was represented as mean value±standard deviation (SD), whereas the non-normal distribution was described as median and quartile (M [Q1, Q3]). Unpaired
Results
A total of 3031 participants were included in this study, with 1091 men (35.99%) and 1940 women (64.01%) (Figure 1). The mean age of the men was 31.17±4.65 years, and the mean age of women, 29.98±4.14 years. The mean age of men was higher than that of women (
As shown in Table 2, the rates of NHcy and HHcy were 66.51% (2016/3031) and 33.91% (1015/3031) in all participants, while they were 33.55% (366/1091) and 66.45% (725/1091) in men, and 85.05% (1650/1940) and 14.95% (290/1940) in women, respectively. HHcy levels were significantly higher in the men than in women (χ2=831.656,
No significant difference was observed in Hcy levels in the male participants aged between 18 and 34 years and in the ≥35 years group (
Hcy levels in the male and female groups were lowest in autumn and highest in winter (
Higher serum folate levels were recorded in the NHcy group than in the HHcy group (
Regression analysis of Hcy levels with sex, age, season, and serum folate level suggested that sex, season, and serum folate levels affected Hcy levels, with serum folate having the greatest effect (standardized coefficient 0.381). Taking winter as a reference, Hcy levels in autumn, summer, and winter were significantly different (
Discussion
In this study, the results indicated that the average Hcy level in men was higher than that in women among the participants preparing for pregnancy (
In the present study, Hcy levels were higher in men than in women. A similar phenomenon has been reported in older adults and adolescents [24–27]. The present study not only confirmed that Hcy has a differential effect on different sexes during the preconception, but also showed that sex impacts the percentage of HHcy, similar to results of a recent Israeli study [28]. It is worth noting that the previous studies found that the sex differences even persisted during the subgroup analysis of participants aged over 55 years, when women experience perimenopause, with lower estrogen levels [24,28]. Therefore, in preconception population care, it is also important to focus on the assessment of men, not just women. However, the cause of the sex-induced Hcy difference is still controversial. Physical activity, endogenous estrogens, rates of homocysteine remethylation, and methylenetetrahydrofolate reductase (MTHFR) C677T genotypes are a few reasons [27,29–32].
Previous studies have suggested that age is a factor affecting Hcy and that Hcy levels increase each year, with the highest levels being found in elderly people (75 years or older) [33]. Also, age significantly affects Hcy metabolism dynamics in the pediatric population [34]. In the present study, age showed a significant difference in Hcy levels in women between 18 and 34 years old and ≥35 years old, before the confounders were adjusted. However, further multivariate regression analysis revealed that age was not a standalone risk factor for Hcy, which is different from the results indicated in earlier studies. The reason age was not associated with Hcy levels in the preconception population is puzzling. Xu et al [24] found that Hcy levels in a Chinese population initially increase before decreasing, with the trend being generally constant from 30 to 50 years of age, and then levels significantly increase beyond 50 years of age. However, another study comparing White, Black, and Hispanic populations in the United States revealed that Hcy levels increase with increasing age throughout adulthood and differ by race-ethnicity [35]. Therefore, race may be the reason for the unrelated relationship between age and Hcy in this Chinese preconception population.
Surprisingly, Hcy levels varied with changing seasons. The levels of Hcy in men and women were lowest during the fall season and highest in winter. Recently, studies showed an inverse relationship between vitamin D levels and Hcy [36,37]. Also, Al-Bayyari et al [38] observed that vitamin D3 therapy remarkably lowered Hcy levels. According to several research reports, individuals have higher serum vitamin D concentrations in summer and fall than in winter and spring [37,39,40]. Therefore, we speculated that vitamin D was one of the reasons for the seasonal differences in Hcy levels. However, the relationship between Hcy and seasons is controversial. Similar to the present study, Clarke et al [41] found the median Hcy was 3% higher in summer than in winter. Meanwhile Checinska [42] found that cold-water swimmers have lower Hcy levels in winter, and Bates et al [43] observed that people aged 4 to 18 years and those aged 65 years and older often showed the lowest mean Hcy levels. In contrast, Michelle et al [44] showed there was no seasonal variation in plasma total Hcy levels. These inconsistent results may be due to differences in the geographic, racial, and social characteristics of participants. Therefore, further investigation needs to be conducted to validate the results of the present study. Nevertheless, the results of this study are still important for reminding preconception-care physicians to pay attention to the Hcy levels of couples preparing for pregnancy in winter.
Hcy is produced during methionine metabolism, and a proportion of Hcy binds to serine and creates cystathionine; however, most Hcy molecules are remethylated to produce methionine. This process requires plenty of reduced folate and a proper MTHFR effect [1].Therefore, folate metabolism is closely related to Hcy metabolism. Many previous studies have suggested that folate deficiency is strongly associated with HHcy [45–47]. The results of the present study confirmed the earlier findings, specifically, whereby the HHcy group had a lower serum folate level. At present, there is a consensus that folic acid is one of the most common and effective supplements used to lower plasma Hcy levels [48,49]. Daily supplementation of 0.5 to 5.0 mg of folic acid typically lowers plasma Hcy levels by approximately 25% [50]. In one study, 56.41% of patients with HHcy reach the normal range (5–15 mmol/L) after 3 months of folic acid supplementation [51]. Therefore, it is important to take folic acid supplements 3 to 6 months before pregnancy.
This study had a few limitations. First, in this retrospective study, the preconception population who had received folic acid and vitamin D in advance were not excluded, which may have affected the levels of Hcy, leading to bias in the study. Second, this study lacked information about red blood cell folate, which, as the long-term indicator of folate status, is frequently used to assess folate status [52]. Third, Hcy levels are influenced by food groups to varying degrees [53], and Hcy levels are decreased under a low-protein diet and Mediterranean diet [54,55]; however, this study did not include an analysis of the participants’ eating habits. Fourth, there was just one data center used in this investigation.
Conclusions
In this retrospective study from a single center, we aimed to investigate the distribution of plasma Hcy levels in a preconception population and further evaluate the factors influencing plasma Hcy levels in Southern China. The findings showed that Hcy levels were higher in men and in winter. Sex, season, and serum folate levels were the influencing factors of Hcy levels in this preconception population.
Tables
Table 1. Comparison of age and homocysteine (Hcy) levels in the preconception population by sex.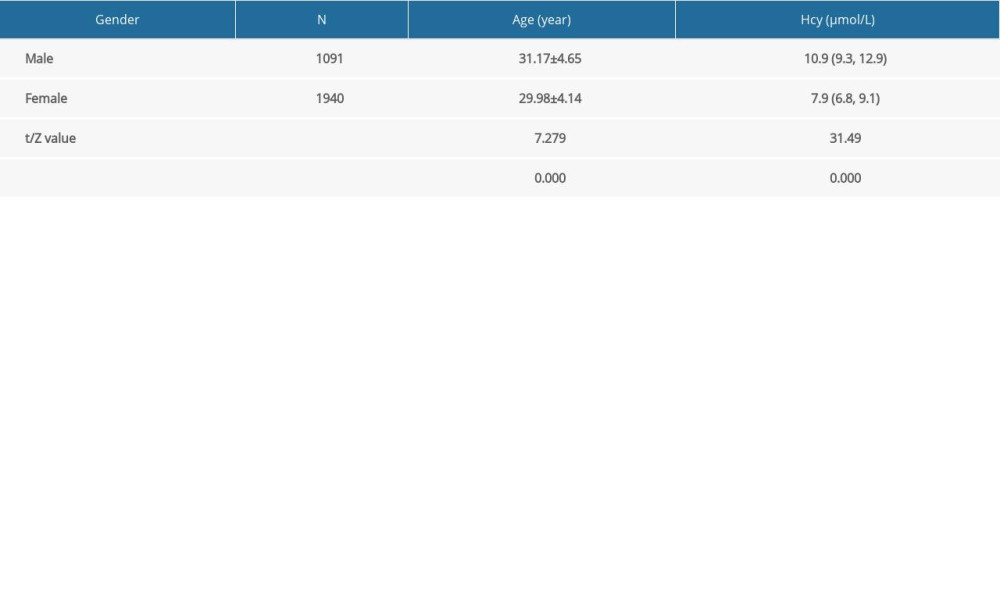 Table 2. Normal homocysteine (NHcy) and hyperhomocysteinemia (HHcy) detection rates in the preconception population by sex.
Table 2. Normal homocysteine (NHcy) and hyperhomocysteinemia (HHcy) detection rates in the preconception population by sex.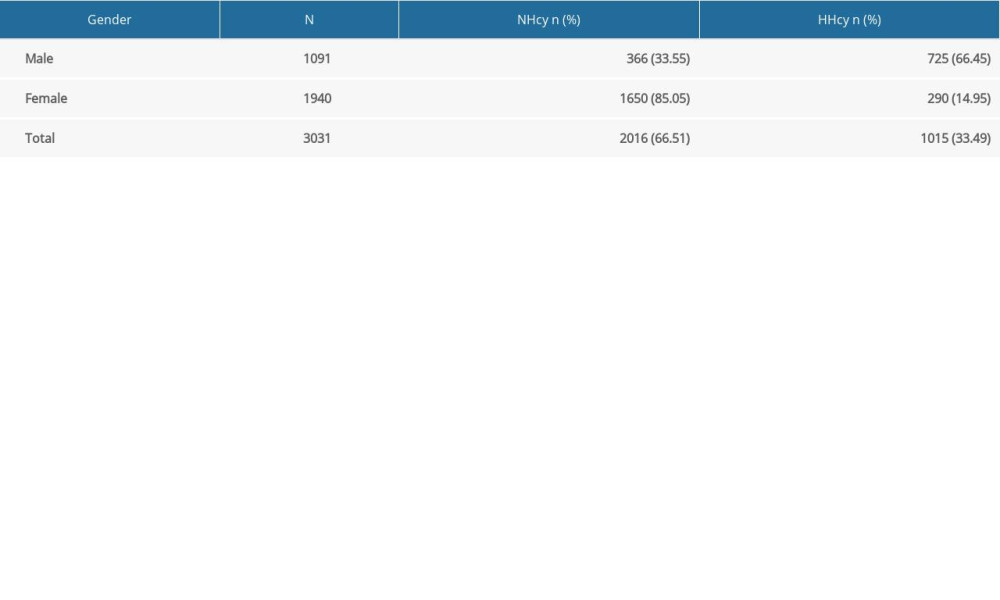 Table 3. Comparison of homocysteine (Hcy) levels in the preconception population by sex and age.
Table 3. Comparison of homocysteine (Hcy) levels in the preconception population by sex and age.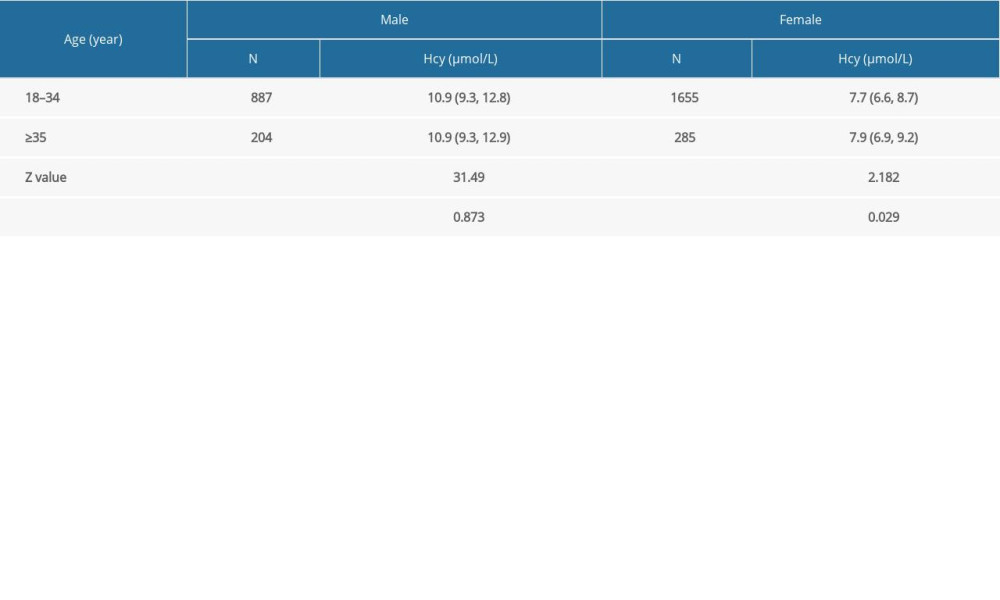 Table 4. Comparison of homocysteine (Hcy) levels in the preconception population by sex and season.
Table 4. Comparison of homocysteine (Hcy) levels in the preconception population by sex and season.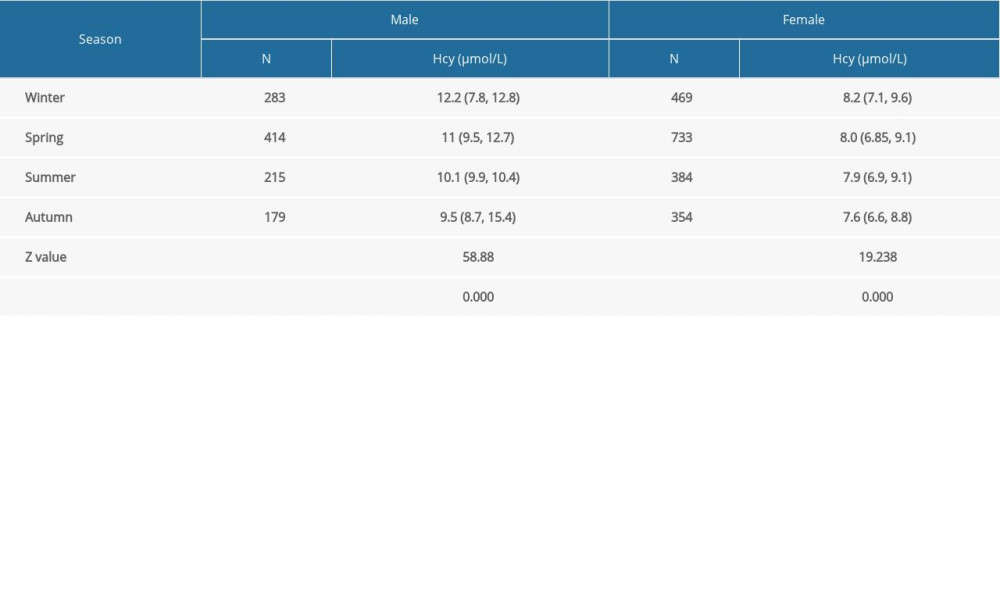 Table 5. Comparison of serum folate levels in the preconception population by sex and homocysteine (Hcy) level.
Table 5. Comparison of serum folate levels in the preconception population by sex and homocysteine (Hcy) level.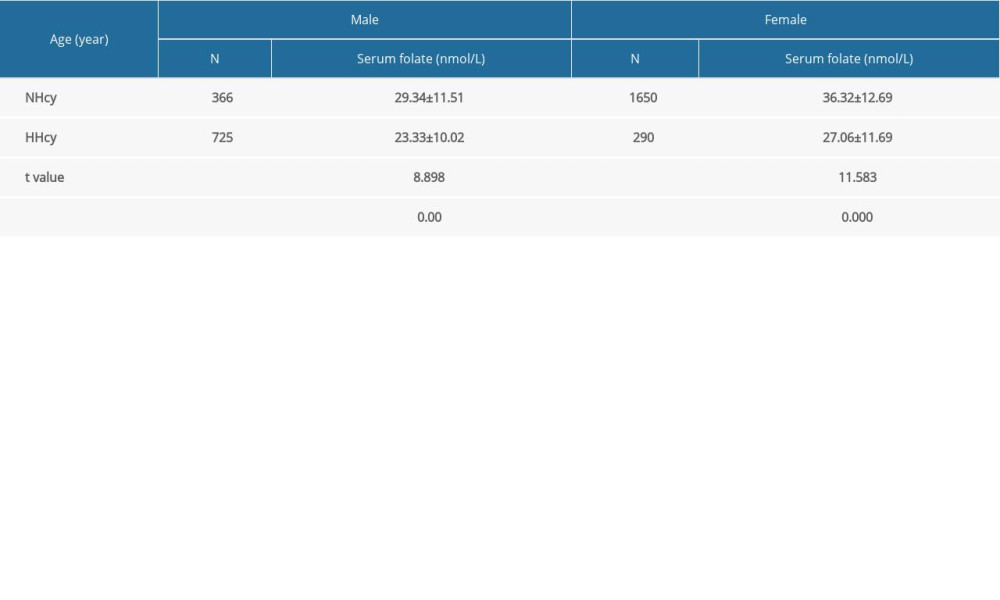 Table 6. Regression analysis of the Hcy level with gender, age, serum folate level, and season.
Table 6. Regression analysis of the Hcy level with gender, age, serum folate level, and season.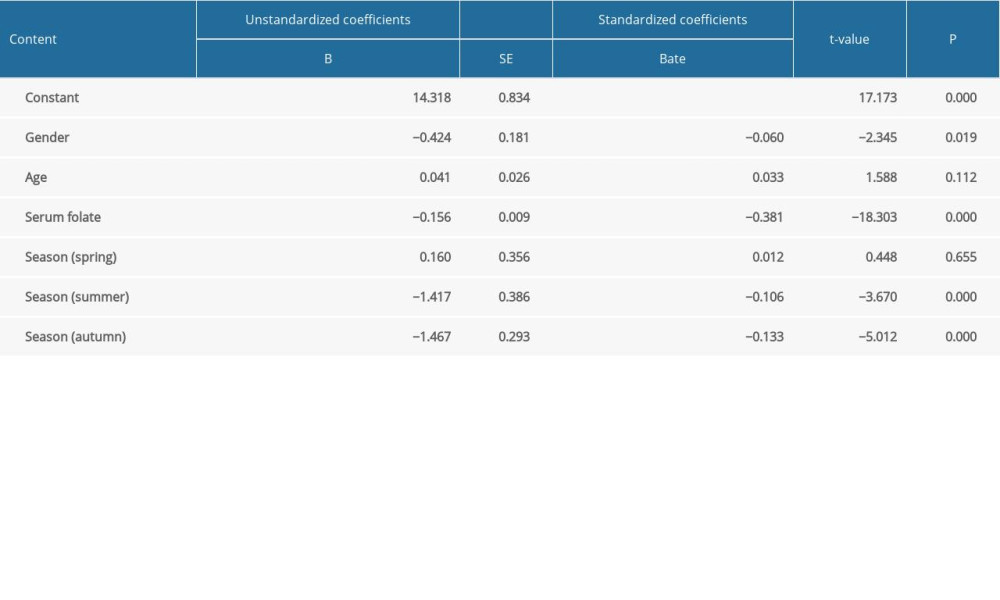
References
1. Dai C, Fei Y, Li J, A novel review of homocysteine and pregnancy complications: Biomed Res Int, 2021; 2021; 6652231
2. Wyse ATS, Bobermin LD, Dos Santos TM, Quincozes-Santos A, Homocysteine and gliotoxicity: Neurotox Res, 2021; 39(3); 966-74
3. Shen W, Gao C, Cueto R, Homocysteine-methionine cycle is a metabolic sensor system controlling methylation-regulated pathological signaling: Redox Biol, 2020; 28; 101322
4. Smith AD, Refsum H, Homocysteine – from disease biomarker to disease prevention: J Int Med, 2021; 290(4); 826-54
5. Guieu R, Ruf J, Mottola G, Hyperhomocysteinemia and cardiovascular diseases: Ann Biol Clin, 2022; 80(1); 7-14
6. Ji Y, Lyu P, Jin W, Homocysteine: A modifiable culprit of cognitive impairment for us to conquer?: J Neurol Sci, 2019; 404; 128-36
7. Poddar R, Hyperhomocysteinemia is an emerging comorbidity in ischemic stroke: Exp Neurol, 2021; 336; 113541
8. Mu L, Yu F, Xia J, Association between high BMI and high homocysteine levels in Chinese patients with bipolar disorder: J Affect Disord, 2021; 295; 284-90
9. Wu F, Yang H, Liu B, Association between homocysteine and arterial stiffness in women with a history of preeclampsia: J Vasc Res, 2019; 56(3); 152-59
10. Sacco RL, Adams R, Albers G, Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: A statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co-sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline: Circulation, 2006; 113(10); e409-49
11. Fan R, Zhang A, Zhong F, Association between homocysteine levels and all-cause mortality: A dose-response meta-analysis of prospective studies: Sci Rep, 2017; 7(1); 4769
12. Hofmann MA, Lalla E, Lu Y, Hyperhomocysteinemia enhances vascular inflammation and accelerates atherosclerosis in a murine model: J Clin Invest, 2001; 107(6); 675-83
13. Papatheodorou L, Weiss N, Vascular oxidant stress and inflammation in hyperhomocysteinemia: Antiox Redox Sign, 2007; 9(11); 1941-58
14. Chaudhry SH, Taljaard M, MacFarlane AJ, The role of maternal homocysteine concentration in placenta-mediated complications: Findings from the Ottawa and Kingston birth cohort: BMC Pregnancy Childbirth, 2019; 19(1); 75
15. Wadhwani NS, Patil VV, Mehendale SS, Increased homocysteine levels exist in women with preeclampsia from early pregnancy: J Matern Fetal Neonatal Med, 2016; 29(16); 2719-25
16. Kasture VV, Sundrani DP, Joshi SR, Maternal one carbon metabolism through increased oxidative stress and disturbed angiogenesis can influence placental apoptosis in preeclampsia: Life Sci, 2018; 206; 61-69
17. Knight AK, Park HJ, Hausman DB, Association between one-carbon metabolism indices and DNA methylation status in maternal and cord blood: Sci Rep, 2018; 8(1); 16873
18. Gaiday AN, Tussupkaliyev AB, Bermagambetova SK, Effect of homocysteine on pregnancy: A systematic review: Chem Biol Interact, 2018; 293; 70-76
19. Bala R, Verma R, Verma P, Hyperhomocysteinemia and low vitamin B12 are associated with the risk of early pregnancy loss: A clinical study and meta-analyses: Nutr Res, 2021; 91; 57-66
20. Qin W, Hu X, Fu C, Estimation of homocysteine concentration as an indicator of foetal death in pregnant Chinese women with preeclampsia: A case-control study: J Clin Lab Anal, 2022; 36(4); e24312
21. Walker MC, Smith GN, Perkins SL, Changes in homocysteine levels during normal pregnancy: Am J Obstet Gynecol, 1999; 180(3 Pt 1); 660-64
22. World Health Organization: Meeting to develop a global consensus on preconception care to reduce maternal and childhood mortality and morbidity: World Health Organization Headquarters, Geneva, 6–7 February 2012 meeting report, 2013 Available from https://apps.who.int/iris/handle/10665/78067
23. Tu W, Yan F, Chao B, Ji X, Wang L, Status of hyperhomocysteinemia in China: Results from the China Stroke High-risk Population Screening Program, 2018: Front Med, 2021; 15(6); 903-12
24. Xu R, Huang F, Wang Y, Gender- and age-related differences in homocysteine concentration: A cross-sectional study of the general population of China: Sci Rep, 2020; 10(1); 17401
25. Dankner R, Chetrit A, Lubin F, Sela BA, Life-style habits and homocysteine levels in an elderly population: Aging Clin Exp Res, 2004; 16(6); 437-42
26. Wang F, Sui X, Xu N, The relationship between plasma homocysteine levels and MTHFR gene variation, age, and sex in Northeast China: Niger J Clin Pract, 2019; 22(3); 380-85
27. Xiang T, Xiang H, Yan M, Systemic risk factors correlated with hyperhomocysteinemia for specific MTHFR C677T genotypes and sex in the Chinese population: Ann Transl Med, 2020; 8(21); 1455
28. Cohen E, Margalit I, Shochat T, Gender differences in homocysteine concentrations, a population-based cross-sectional study: Nutr Metab Cardiovasc Dis, 2019; 29(1); 9-14
29. Loprinzi PD, Cardinal BJ, Interrelationships among physical activity, depression, homocysteine, and metabolic syndrome with special considerations by sex: Prevent Med, 2012; 54(6); 388-92
30. Christodoulakos GE, Lambrinoudaki IV, Rizos DA, Endogenous sex steroids and circulating homocysteine in healthy Greek postmenopausal women: Int J Endocrinol Metab, 2006; 5(1); 35-41
31. Lakryc EM, Machado RB, Soares JM, What is the influence of hormone therapy on homocysteine and crp levels in postmenopausal women?: Clinics, 2015; 70(2); 107-13
32. Fukagawa NK, Martin JM, Wurthmann A, Sex-related differences in methionine metabolism and plasma homocysteine concentrations: Am J Clin Nutr, 2000; 72(1); 22-29
33. Ostrakhovitch EA, Tabibzadeh S, Homocysteine and age-associated disorders: Ageing Res Rev, 2019; 49; 144-64
34. Caldeira-Araújo H, Ramos R, Homocysteine metabolism in children and adolescents: influence of age on plasma biomarkers and correspondent genotype interactions: Nutrients, 2019; 11(3); 646
35. Jacques PF, Rosenberg IH, Rogers G, Serum total homocysteine concentrations in adolescent and adult Americans: Results from the third National Health and Nutrition Examination Survey: Am J Clin Nutr, 1999; 69(3); 482-89
36. Ota K, Takahashi T, Han A, Damvaeba S, Effects of MTHFR C677T polymorphism on vitamin D, homocysteine and natural killer cell cytotoxicity in women with recurrent pregnancy losses: Human Reprod, 2020; 35(6); 1276-87
37. Glueck CJ, Jetty V, Rothschild M, Associations between serum 25-hydroxyvitamin D and lipids, lipoprotein cholesterols, and homocysteine: North Am J Med Sci, 2016; 8(7); 284-90
38. Al-Bayyari N, Al-Zeidaneen S, Hailat R, Hamadneh J, Vitamin D3 prevents cardiovascular diseases by lowering serum total homocysteine concentrations in overweight reproductive women: A randomized, placebo-controlled clinical trial: Nutr Res, 2018; 59; 65-71
39. Perreault M, Atkinson SA, Meyre D, Summer season and recommended vitamin D intake support adequate vitamin D status throughout pregnancy in healthy Canadian women and their newborns: J Nutr, 2020; 150(4); 739-46
40. Gu Y, Zhu Z, Luan X, He J, Vitamin D status and its association with season, depression in stroke: Neurosci Lett, 2019; 690; 99-105
41. Clarke R, Woodhouse P, Ulvik A, Variability and determinants of total homocysteine concentrations in plasma in an elderly population: Clin Chem, 1998; 44(1); 102-7
42. Checinska-Maciejewska Z, Miller-Kasprzak E, Checinska A, Gender-related effect of cold water swimming on the seasonal changes in lipid profile, ApoB/ApoA-I ratio, and homocysteine concentration in cold water swimmers: J Physiol Pharmacol, 2017; 68(6); 887-96
43. Bates CJ, Mansoor MA, Gregory J, Correlates of plasma homocysteine, cysteine and cysteinyl-glycine in respondents in the British National Diet and Nutrition Survey of young people aged 4–18 years, and a comparison with the survey of people aged 65 years and over: Br J Nutr, 2002; 87(1); 71-79
44. McKinley MC, Strain JJ, McPartlin J, Plasma homocysteine is not subject to seasonal variation: Clin Chem, 2001; 47(8); 1430-36
45. Kim J, Kim H, Roh H, Kwon Y, Causes of hyperhomocysteinemia and its pathological significance: Arch Pharm Res, 2018; 41(4); 372-83
46. Blom HJ, Smulders Y, Overview of homocysteine and folate metabolism. With special references to cardiovascular disease and neural tube defects: J Inherit Metab Dis, 2011; 34(1); 75-81
47. Stanhewicz AE, Kenney WL, Role of folic acid in nitric oxide bioavailability and vascular endothelial function: Nutr Rev, 2017; 75(1); 61-70
48. Brouwer IA, van Dusseldorp M, Thomas CM, Low-dose folic acid supplementation decreases plasma homocysteine concentrations: A randomized trial: Am J Clin Nutr, 1999; 69(1); 99-104
49. Homocysteine Lowering Trialists’ Collaboration, Dose-dependent effects of folic acid on blood concentrations of homocysteine: A meta-analysis of the randomized trials: Am J Clin Nutr, 2005; 82(4); 806-12
50. Kaye AD, Jeha GM, Pham AD, Folic acid supplementation in patients with elevated homocysteine levels: Adv Ther, 2020; 37(10); 4149-64
51. Tian H, Tian D, Zhang C, Efficacy of folic acid therapy in patients with hyperhomocysteinemia: J Am Coll Nutr, 2017; 36(7); 528-32
52. World Health Organization: Guideline: Optimal serum and red blood cell folate concentrations in women of reproductive age for prevention of neural tube defects, 2015 Available fromhttps://apps.who.int/iris/handle/10665/161988
53. Zeng Q, Li F, Xiang T, Influence of food groups on plasma total homocysteine for specific MTHFR C677T genotypes in Chinese population: Mol Nutr Food Res, 2017; 61(2); 1600351
54. Deminice R, Portari GV, Marchini JS, Effects of a low-protein diet on plasma amino acid and homocysteine levels and oxidative status in rats: Ann Nutri Metab, 2009; 54(3); 202-7
55. Foscolou A, Rallidis LS, Tsirebolos G, The association between homocysteine levels, Mediterranean diet and cardiovascular disease: A case-control study: Int Food Sci Nutr, 2019; 70(5); 603-11
Tables
 Table 1. Comparison of age and homocysteine (Hcy) levels in the preconception population by sex.
Table 1. Comparison of age and homocysteine (Hcy) levels in the preconception population by sex. Table 2. Normal homocysteine (NHcy) and hyperhomocysteinemia (HHcy) detection rates in the preconception population by sex.
Table 2. Normal homocysteine (NHcy) and hyperhomocysteinemia (HHcy) detection rates in the preconception population by sex. Table 3. Comparison of homocysteine (Hcy) levels in the preconception population by sex and age.
Table 3. Comparison of homocysteine (Hcy) levels in the preconception population by sex and age. Table 4. Comparison of homocysteine (Hcy) levels in the preconception population by sex and season.
Table 4. Comparison of homocysteine (Hcy) levels in the preconception population by sex and season. Table 5. Comparison of serum folate levels in the preconception population by sex and homocysteine (Hcy) level.
Table 5. Comparison of serum folate levels in the preconception population by sex and homocysteine (Hcy) level. Table 6. Regression analysis of the Hcy level with gender, age, serum folate level, and season.
Table 6. Regression analysis of the Hcy level with gender, age, serum folate level, and season. Table 1. Comparison of age and homocysteine (Hcy) levels in the preconception population by sex.
Table 1. Comparison of age and homocysteine (Hcy) levels in the preconception population by sex. Table 2. Normal homocysteine (NHcy) and hyperhomocysteinemia (HHcy) detection rates in the preconception population by sex.
Table 2. Normal homocysteine (NHcy) and hyperhomocysteinemia (HHcy) detection rates in the preconception population by sex. Table 3. Comparison of homocysteine (Hcy) levels in the preconception population by sex and age.
Table 3. Comparison of homocysteine (Hcy) levels in the preconception population by sex and age. Table 4. Comparison of homocysteine (Hcy) levels in the preconception population by sex and season.
Table 4. Comparison of homocysteine (Hcy) levels in the preconception population by sex and season. Table 5. Comparison of serum folate levels in the preconception population by sex and homocysteine (Hcy) level.
Table 5. Comparison of serum folate levels in the preconception population by sex and homocysteine (Hcy) level. Table 6. Regression analysis of the Hcy level with gender, age, serum folate level, and season.
Table 6. Regression analysis of the Hcy level with gender, age, serum folate level, and season. In Press
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952