29 November 2022: Clinical Research
Sex-Related Differences in Sitagliptin Treatment in Type 2 Diabetes: Results from the PROLOGUE Trial
Xilin Wu1ABCDEF, Shiming Zhang1ABCEF, Yingzhu Jin2ABC, Xun Xiao3ABCDEF*DOI: 10.12659/MSM.938030
Med Sci Monit 2022; 28:e938030
Abstract
BACKGROUND: At present, whether sitagliptin has sex-related differences in effect on atherosclerosis in type 2 diabetes mellitus (T2DM) patients is unknown. The purpose of this study was to investigate whether there is sex-related difference in the effect of sitagliptin on atherosclerosis in T2DM patients.
MATERIAL AND METHODS: In the PROLOGUE trial, 222 patients were allocated to the sitagliptin group and 220 patients were allocated to the conventional group. Carotid artery intima-media thickness (IMT) was assessed at baseline, 12 months, and 24 months.
RESULTS: In male patients, sitagliptin significantly reduced the mean IMT (0.84±0.41 mm vs 1.02±0.67 mm, P=0.013) and the maximum IMT (1.14±0.59 mm vs 1.39±0.88 mm, P=0.016) in the right internal carotid arteries (ICA) compared to the conventional group at 12 months. Similarly, sitagliptin significantly reduced the maximum IMT (1.09±0.52 mm vs 1.28±0.77 mm, P=0.049) in the right ICA compared to the conventional group at 24 months, but no difference was found in the mean IMT in the right ICA between groups at 24 months. In female patients, sitagliptin significantly reduced the mean IMT (1.01±0.47 mm vs 1.23±0.51 mm, P=0.049) and the maximum IMT (1.39±0.65 mm vs 1.71±0.77 mm, P=0.042) in the right bulb compared to the conventional group at 12 months. However, the group differences were not observed in mean IMT and maximum IMT at 24 months.
CONCLUSIONS: Our results suggest that sitagliptin slows the progression of right carotid IMT in male patients. However, more research is needed to validate this finding in female patients.
Keywords: 1,5,5-Triallylbarbituric Acid, 46, XY Sex Reversal 5, 6q24-Related Transient Neonatal Diabetes Mellitus, Sitagliptin Phosphate, Humans, Female, Male, Diabetes Mellitus, Type 2, carotid intima-media thickness, atherosclerosis, Carotid Artery, Internal
Background
Atherosclerosis [1–3] is a chronic non-infectious inflammation of the arterial wall. Previous studies have shown that cardiovascular [4,5] and cerebrovascular [6,7] diseases caused by atherosclerosis are some of the leading causes of death in developed and developing countries. In China, atherosclerosis has become one of the main causes of death [8,9]. Atherosclerosis is caused by a variety of factors, including endothelial cell injury, dyslipidemia, diabetes, hypertension, and chronic inflammation. Among these factors, type 2 diabetes mellitus (T2DM) is a critical risk factor for atherosclerosis [10–12]. There is clinical evidence showing higher risk of coronary heart disease (CHD) [13] and ischemic stroke (IS) [14] in T2DM patients than in patients with a single disease. Therefore, the main focus of research has been to determine ways to delay or reduce the degree of atherosclerosis in T2DM patients.
Dipeptidyl peptidase-4 (DPP-4) inhibitor [15,16] is a new therapeutic drug for the treatment of diabetes. It can elevate the activity of endogenous glucagon-like peptide-1 (GLP-1) and gastric inhibitory polypeptide (GIP), promote the release of insulin by islet B cells, and restrain the excretion of glucagon, thus causing an increase in insulin concentration and a drop in blood glucose. Sitagliptin, one of the inhibitors of DPP-4 inhibitors, has been widely assessed in previous studies [17,18]. However, whether sitagliptin can effectively delay the progression of atherosclerosis in T2DM patients and thus reduce cardiovascular events remains controversial. Some previous studies have found that sitagliptin does not improve the degree of atherosclerosis in T2DM patients [19,20], nor reduce the risk of cardiovascular events [21,22]. On the contrary, some other studies have supported the benefits of sitagliptin in T2DM patients [23–25]. It is likely that these conflicting results are due to sex-related differences, since males and females have different levels of hormone secretion (eg, androgens, estrogens, and progestins), which could potentially impact the effectiveness of sitagliptin treatment in T2DM patients. In addition, there have been no studies reporting sex-related differences in the effect of sitagliptin on carotid artery IMT. Therefore, the objective of our study was to re-analyze the PROLOGUE study, and explore whether sex-related differences impact the effectiveness of sitagliptin treatment in patients with T2DM [19].
Material and Methods
ENDPOINTS:
The primary endpoint of our current study included changes in the mean IMT in the right and the left common carotid arteries (CCA), the mean IMT in the right and the left bulb, the mean IMT in the right and the left internal carotid arteries (ICA), the maximum IMT in the right and the left CCA, the maximum IMT in the right and the left bulb, and the maximum IMT in the right and the left ICA. The primary endpoints were measured by high-resolution carotid ultrasonography at baseline, 12 months, and 24 months by expert sonographers trained in the measurement of carotid IMT, as described in Oyama et al [19]. In addition, all ultrasonic systems were equipped with more than 7.5 MHz linear sensors. The secondary endpoints included weight, waist circumference (WC), systolic blood pressure (SDP), diastolic blood pressure (DBP), pulse rate (PR), HbA1c, fasting plasma glucose (FPG), 1,5-anhydroglucitol,1,4-anhydro-D-glucitol, high-molecular-weight adiponectin (HMWA), lactic dehydrogenase (LD), high-density lipoprotein cholesterol (HDL-cholesterol), triglyceride (TG), small dense low-density lipoprotein cholesterol (small DLD-cholesterol), remnant-like particle cholesterol (RLP-cholesterol), malondialdehyde-modified low-density lipoprotein (MMDLI), aspartate aminotransferase (AST), alanine aminotransferase (ALT), blood urea nitrogen (BUN), serum creatinine, estimated glomerular filtration rate (eGFR), cystatin C, and urinary albumin/creatinine ratio, and were measured at 24 months.
STATISTICAL ANALYSIS:
The Shapiro-Wilk test was used to judge the normality of continuous variables. In this study, we first divided all participants into 2 groups based on sex, and then explored the effect of sitagliptin on carotid atherosclerosis in T2DM patients. We used mean and standard deviations to represent normally distributed continuous variables, and used the independent-samples
Results
BASELINE CHARACTERISTICS OF MALE PARTICIPANTS:
Table 1 shows the baseline characteristics and baseline laboratory information for male participants. The average age of participants in the sitagliptin and the conventional group was 68.21±9.22 years and 68.66±9.24 years, respectively. No significant differences were observed in height, weight, SBP, DBP, HbA1c, FPG, serum creatinine, eGFR, mean IMT in the right and the left CCA, mean IMT in the right and the left bulb, mean IMT in the right and the left ICA, maximum IMT in the right and the left CCA, maximum IMT in the right and the left bulb, maximum IMT in the right and the left ICA, plaque area, plaque gray-scale median, and history of medication and diseases between the 2 groups (all P>0.05).
BASELINE CHARACTERISTICS OF FEMALE PARTICIPANTS:
Table 2 shows the baseline characteristics and baseline laboratory information for female participants. The average age of participants in the sitagliptin group and the conventional group was 71.04±9.10 years and 71.35±8.83 years, respectively. Similar to male participants, no significant differences were observed in height, weight, SBP, DBP, HbA1c, FPG, serum creatinine, eGFR, mean IMT in the right and the left CCA, mean IMT in the right and the left bulb, mean IMT in the right and the left ICA, maximum IMT in the right and the left CCA, maximum IMT in the right and the left bulb, maximum IMT in the right and the left ICA, plaque area, plaque gray scale median, and history of medication and diseases between the 2 groups (all P>0.05).
COMPARISON OF ATHEROSCLEROTIC PARAMETERS IN MALE PARTICIPANTS AT 12 MONTHS AND 24 MONTHS:
Our results show that in male patients, sitagliptin significantly reduced mean IMT (0.84±0.41 mm vs 1.02±0.67 mm, group differences: −0.19, −0.34 to −0.04) (Figure 1A) and maximum IMT (1.14±0.59 mm vs 1.39±0.88 mm, group differences: −0.25, −0.45 to −0.05) in the right ICA (Figure 1B) compared to the conventional group at 12 months. Similarly, sitagliptin also significantly reduced maximum IMT in the right ICA (1.09±0.52 mm vs 1.28±0.77 mm, group differences: −0.19, −0.37 to −0.00) (Figure 1C) compared to the conventional group at 24 months, but showed no difference in the mean IMT in the right ICA between the groups at 24 months. For other atherosclerosis parameters, no difference was found between groups (all P>0.05) (Table 3).
COMPARISON OF ATHEROSCLEROTIC PARAMETERS IN FEMALE PARTICIPANTS AT 12 MONTHS AND 24 MONTHS:
For female patients, our results also showed that sitagliptin significantly reduced the mean IMT (1.01±0.47 mm vs 1.23±0.51 mm, P=0.049) and the maximum IMT (1.39±0.65 mm vs 1.71±0.77 mm, P=0.042) in the right bulb compared to the conventional group at 12 months (Figure 2). However, the difference between these 2 indexes was not significant at 24 months. Similarly, no significant difference was noted for other atherosclerosis indicators (all P>0.05) (Table 4).
COMPARISON OF BLOOD LIPID, BLOOD GLUCOSE, AND OTHER INDEXES IN MALE PARTICIPANTS AT 24 MONTHS:
Our results found that in male patients, sitagliptin did not remarkably reduce the levels of blood lipids and blood glucose in the diabetic patients compared to the control group. Blood lipid and blood glucose-associated indices included high-density lipoprotein cholesterol, triglyceride, small dense low-density lipoprotein cholesterol, remnant-like particle cholesterol, malondialdehyde-modified low-density lipoprotein, HbA1c, and fasting plasma glucose, except for high-molecular-weight adiponectin. Similarly, there were no significant inter-group differences in other indicators, including weight, waist circumference, SBP, DBP, pulse rate, 1,5-anhydroglucitol,1,4-anhydro-D-glucitol, lactic dehydrogenase, AST, ALT, BUN, serum creatinine, eGFR, cystatin C, urinary albumin/creatinine ratio, and uric acid (Table 5).
COMPARISON OF BLOOD LIPID, BLOOD GLUCOSE, AND OTHER INDEXES IN FEMALE PARTICIPANTS AT 24 MONTHS:
Our results demonstrated that sitagliptin significantly improved HbA1c levels in female participants compared to the control group (6.52±0.50 vs 6.83±0.82, P=0.010). However, sitagliptin had no impact on blood lipid levels between the groups. Blood lipid-associated indices included high-molecular-weight adiponectin, high-density lipoprotein cholesterol, triglyceride, small dense low-density lipoprotein cholesterol, remnant-like particle cholesterol, and malondialdehyde-modified low-density lipoprotein. Similarly, sitagliptin also had no significant impact on either group for other indicators, including weight, WC, SBP, DBP, pulse rate, FPG, 1,5-anhydroglucitol,1,4-anhydro-D-glucitol, lactic dehydrogenase, AST, ALT, BUN, serum creatinine, eGFR, cystatin C, urinary albumin/creatinine ratio, and uric acid (Table 6).
Discussion
To the best of our knowledge, our study is the first to analyze a randomized controlled trial to investigate sex-related differences in sitagliptin treatment in carotid atherosclerosis in T2DM patients based on the PROLOGUE trial [19]. The main results of this study are as follows: (1) for male patients, sitagliptin slows the progression of right carotid IMT in male patients at both 12 and 24 months. However, this effect of sitagliptin appears to be pronounced only in the right ICA, but this influence was not found in other vessels. In terms of blood glucose and blood lipids, sitagliptin only reduced the level of HMWA and had no significant effect on HDL-cholesterol or HbA1c or FPG. (2) For female patients, sitagliptin could also significantly delay the progression of atherosclerosis, but the effect only occurred at 12 months and did not last until 24 months. This effect seemed to be significant only on the right side of the bulb, but this difference was remarkable in other blood vessels. In addition, our results also show that sitagliptin significantly reduced HbA1c levels at 24 months but had no significant effect on blood lipids and renal function.
In clinical practice, IMT of the carotid artery is often measured by ultrasonic examination [26,27], which then allows clinicians to predict and estimate the risk of cardiovascular events and stroke and to develop treatment strategies. Previous studies have shown a positive correlation between carotid artery IMT and cardiovascular events [28,29], irrespective of whether the patients have cardiovascular disease or not. In addition, ultrasonic examination is non-invasive, making it a safe measure to assess cardiovascular events and stroke. For these reasons, the American Heart Association assesses cardiovascular risk by measuring carotid artery IMT, which is an important indicator of cardiovascular risk [30]. The PROLOGUE study [19] measured carotid artery IMT 3 times, and measured multiple arteries, providing enough samples to show statistically significant differences between the groups. Therefore, we had valid reasons to use carotid artery IMT as the primary endpoint in this study.
Previous studies have demonstrated that the use of DPP-4 inhibitors in diabetes patients could significantly reduce the degree of atherosclerosis [23–25] and better control the level of blood glucose [31]. In addition, some clinical trials [32,33] supported the finding that DPP-4 inhibitors have beneficial effects on endothelial function in diabetes patients. However, no prior study looked at the impact of sex-related differences on sitagliptin’s effect on atherosclerosis in T2DM patients. In the present study, we compared the effect of sitagliptin on atherosclerosis between male and female diabetes patients. The results showed that sitagliptin delayed the progression of atherosclerosis in male patients, with benefits significant at both 12 and 24 months. On the other hand, in female patients, sitagliptin delayed the progression of atherosclerosis, but with benefits only significant at 12 months. The PROLOGUE study [19] found no evidence to support the effectiveness of sitagliptin in delaying atherosclerosis in diabetic patients. However, it should be emphasized that in the PROLOGUE study, the authors did not stratify the study groups based on sex; therefore, the results reflected the effectiveness of sitagliptin in all patients overall. In addition, the PROLOGUE study did not distinguish between changes in left and right carotid atherosclerosis. This may potentially explain the discrepancies between the results from the PROLOGUE study and our study. Our findings suggest that sitagliptin only significantly benefits the right ICA in male patients and right bulb in female patients. One unanticipated finding was that sitagliptin had different effects on different carotid arteries, which is different from previous studies. In a cohort study [34] of 1414 participants, the authors compared the left and right carotid atherosclerotic plaques and assessed the degree of carotid atherosclerosis using magnetic resonance imaging (MRI). The results showed that the size and composition of the left and the right carotid atherosclerotic plaques were asymmetrically distributed. Left carotid plaque is prone to intra-plaque bleeding (9.1% vs 5.9%), while right carotid plaque is more prone to calcification (37.4% vs 31.6%). Similarly, Katranas et al [35] also found that the left and the right coronary arteries have significant differences in hemorheology and geometric parameters. Therefore, we speculate that differences in anatomical structures or hemodynamics may also lead to different effects of sitagliptin on different carotid atheroscleroses. Presently, there is no study directly clarifying the difference in carotid artery IMT between males and females treated with sitagliptin. The possible mechanism is that the secretion of estrogen and progesterone in postmenopausal women decreases, thus offsetting the benefits of sitagliptin treatment, but this phenomenon does not exist in men. However, more research is needed to further clarify this hypothesis.
Our study also found that there are sex-specific differences in the effects of sitagliptin on blood glucose and blood lipids. Sitagliptin reduced the level of high-molecular-weight adiponectin in male patients, while it could only reduce HbA1c levels in female patients. No significant differences were observed in weight control, waist circumference, and renal function protection in males or females, which is contrary to the previous results. In a retrospective cohort study [36] of 220 patients with type 2 diabetes, the authors found that weight and HbA1c level decreased significantly after 6 months of sitagliptin treatment. Similarly, Kawasaki et al [37] investigated whether sitagliptin could improve renal function in diabetic patients, and found that the glomerular filtration rate (β=0.20,
Our study has several advantages. First, we explored sex-related differences in sitagliptin treatment in T2DM patients for the first time, and showed that the effect of sitagliptin on atherosclerosis in male T2DM patients is higher than that in female T2DM patients, which suggests that sex-related differences are cause different therapeutic effects. Second, we used the parameters of multiple carotid arteries as the primary endpoint and found that sitagliptin had different effects on different carotid arteries, which suggests that the appropriate outcome should be selected in clinical research to reduce selection bias. This study also has some shortcomings. First, the research object of this study was a Japanese population. Therefore, whether the conclusions are applicable to other populations still needs to be assessed. Second, based on the current data, we cannot explore the mechanism of sitagliptin only acting on some carotid arteries, nor can we accurately analyze the reasons for the decrease of HbA1c level without significant difference in the degree of atherosclerosis. Third, information on other drugs (eg, antidiabetic drugs, insulin, statin) in the ROLOGUE study is lacking and may potentially affect final conclusions. Fourth, we found a significant difference in age between males and females (68.44±9.22 vs 71.19±8.94,
Conclusions
Our results suggest that sitagliptin slows the progression of right carotid IMT in male patients. However, more studies are needed to confirm this conclusion in female patients.
Figures
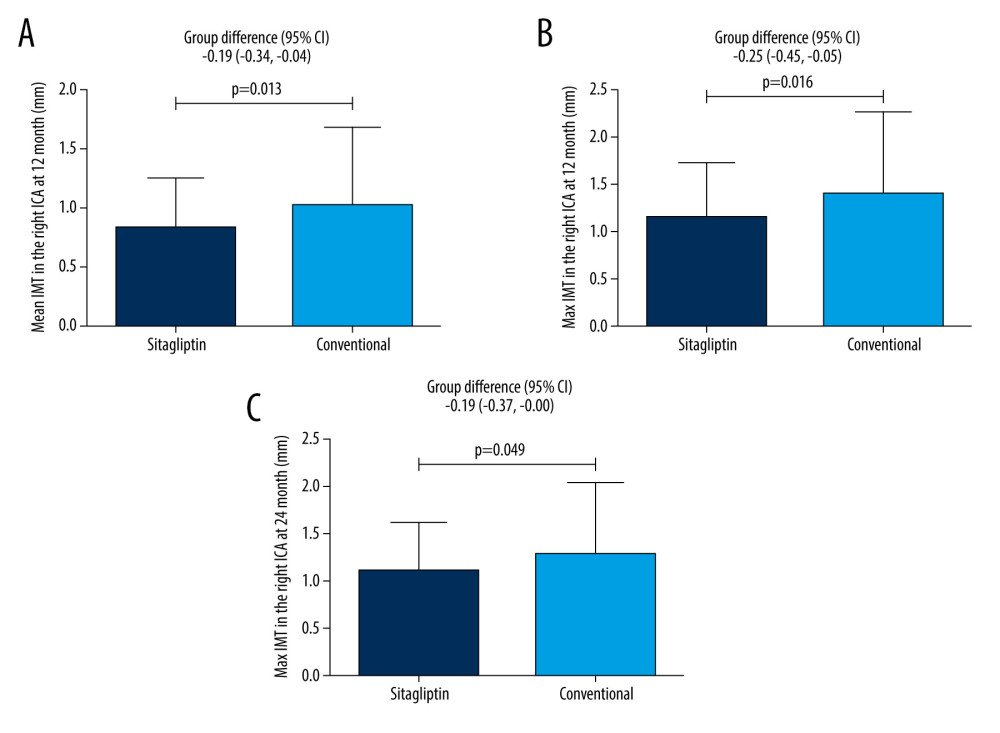 Figure 1. Male patients. (A) Comparison of mean IMT in the right ICA between the 2 groups at 12 months. (B) Comparison of maximum IMT in the right ICA between the 2 groups at 12 months. (C) Comparison of maximum IMT in the right ICA between the 2 groups at 24 months.
Figure 1. Male patients. (A) Comparison of mean IMT in the right ICA between the 2 groups at 12 months. (B) Comparison of maximum IMT in the right ICA between the 2 groups at 12 months. (C) Comparison of maximum IMT in the right ICA between the 2 groups at 24 months. 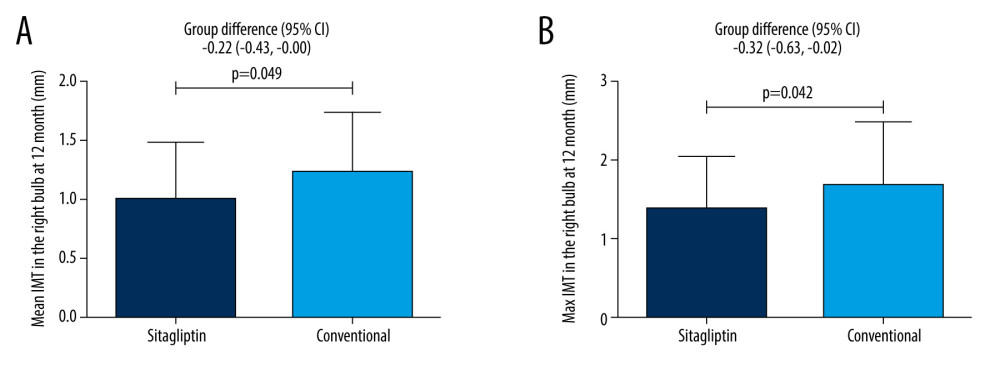 Figure 2. Female patients. (A) Comparison of mean IMT in the right bulb between the 2 groups at 12 months. Figure (B) Comparison of maximum IMT in the right bulb between the 2 groups at 12 months.
Figure 2. Female patients. (A) Comparison of mean IMT in the right bulb between the 2 groups at 12 months. Figure (B) Comparison of maximum IMT in the right bulb between the 2 groups at 12 months. Tables
Table 1. Baseline characteristics of male participants.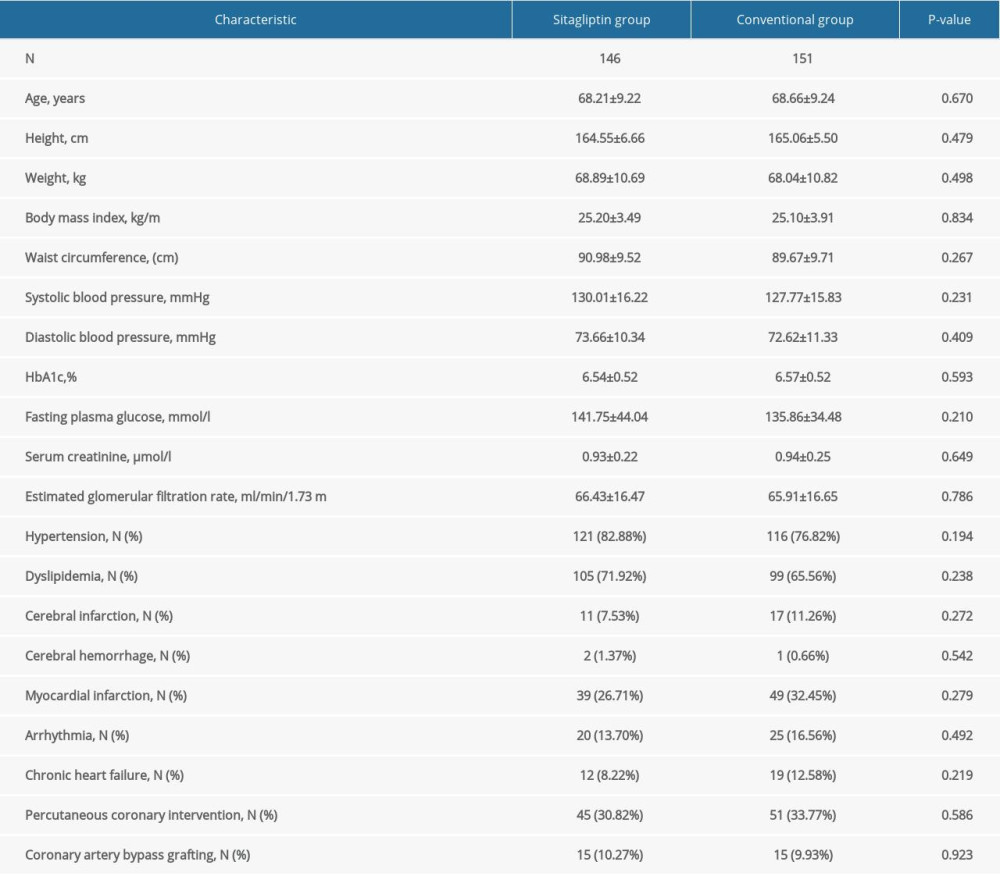 Table 2. Baseline characteristics of female participants.
Table 2. Baseline characteristics of female participants.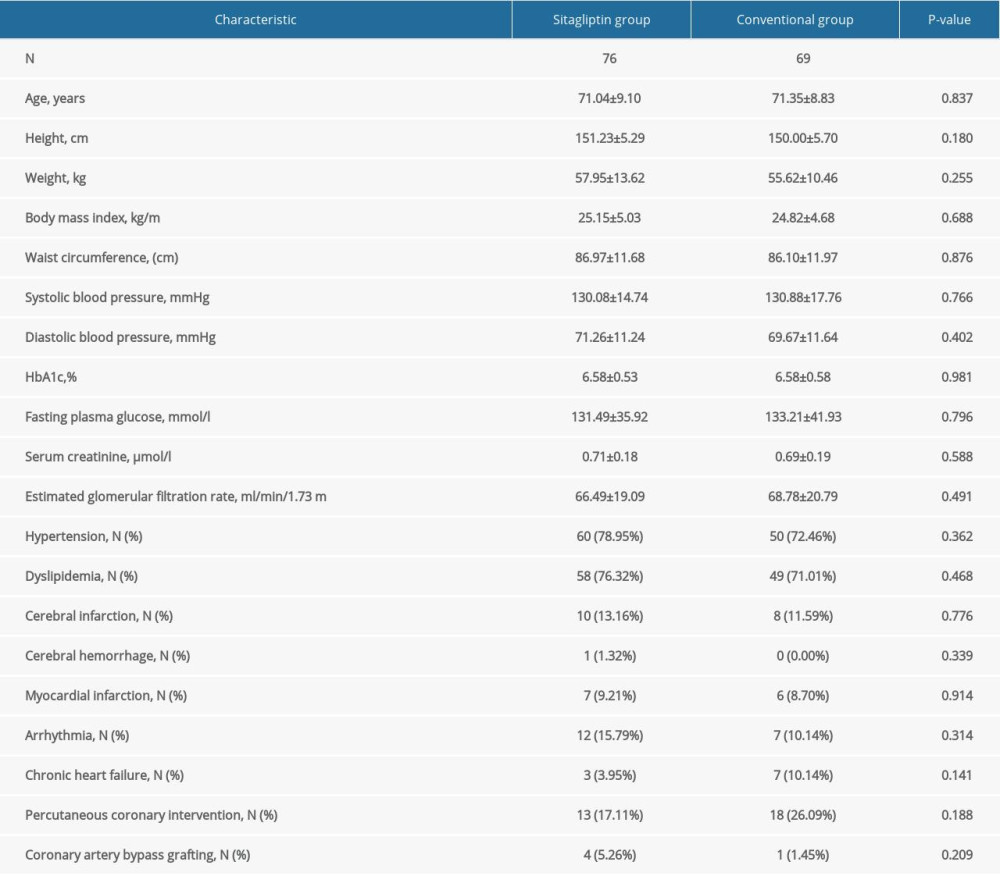 Table 3. Comparison of atherosclerotic parameters at baseline, 12 months, and 24 months in male participants.
Table 3. Comparison of atherosclerotic parameters at baseline, 12 months, and 24 months in male participants.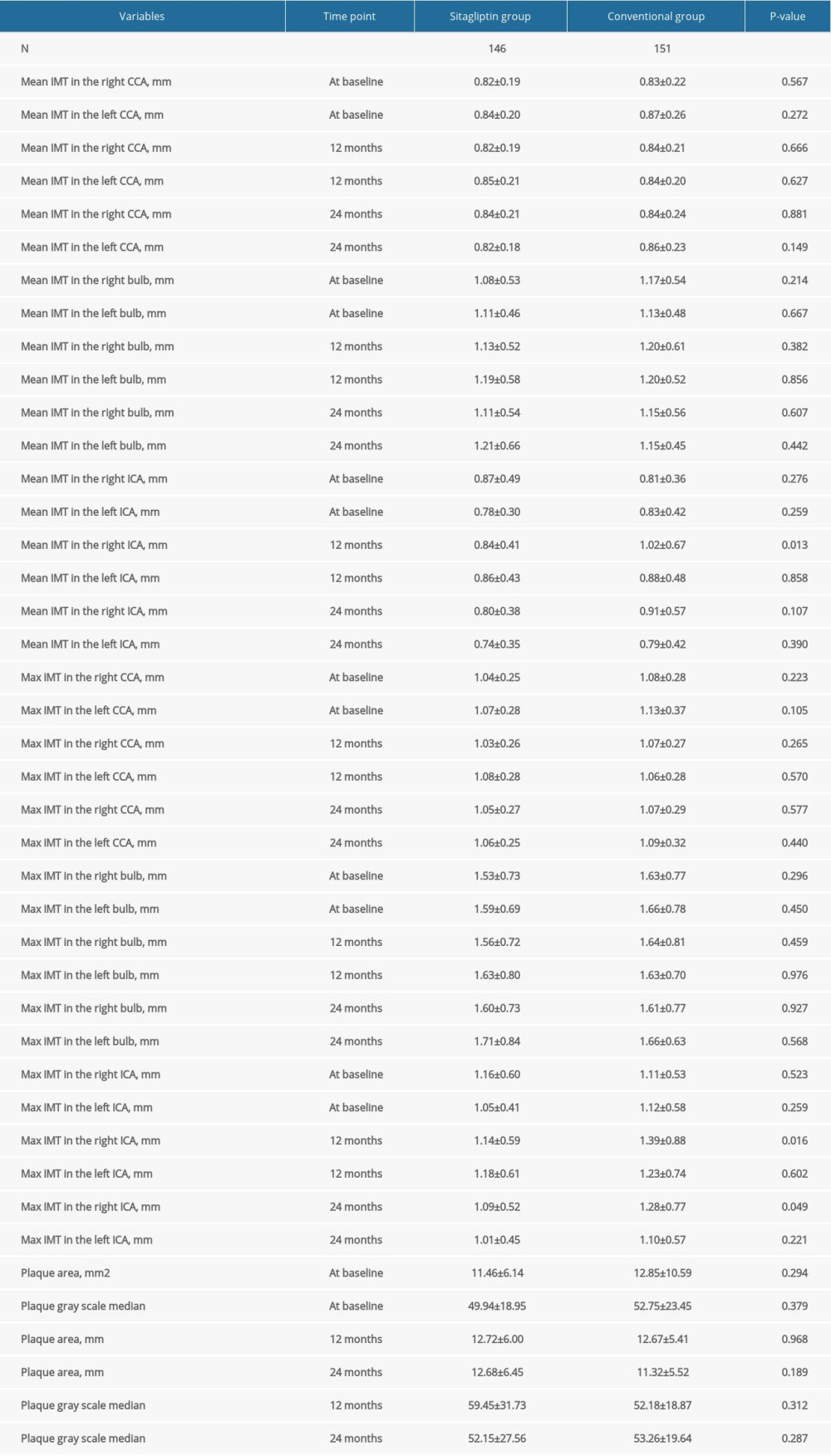 Table 4. Comparison of atherosclerotic parameters at baseline, 12 months, and 24 months in female participants.
Table 4. Comparison of atherosclerotic parameters at baseline, 12 months, and 24 months in female participants.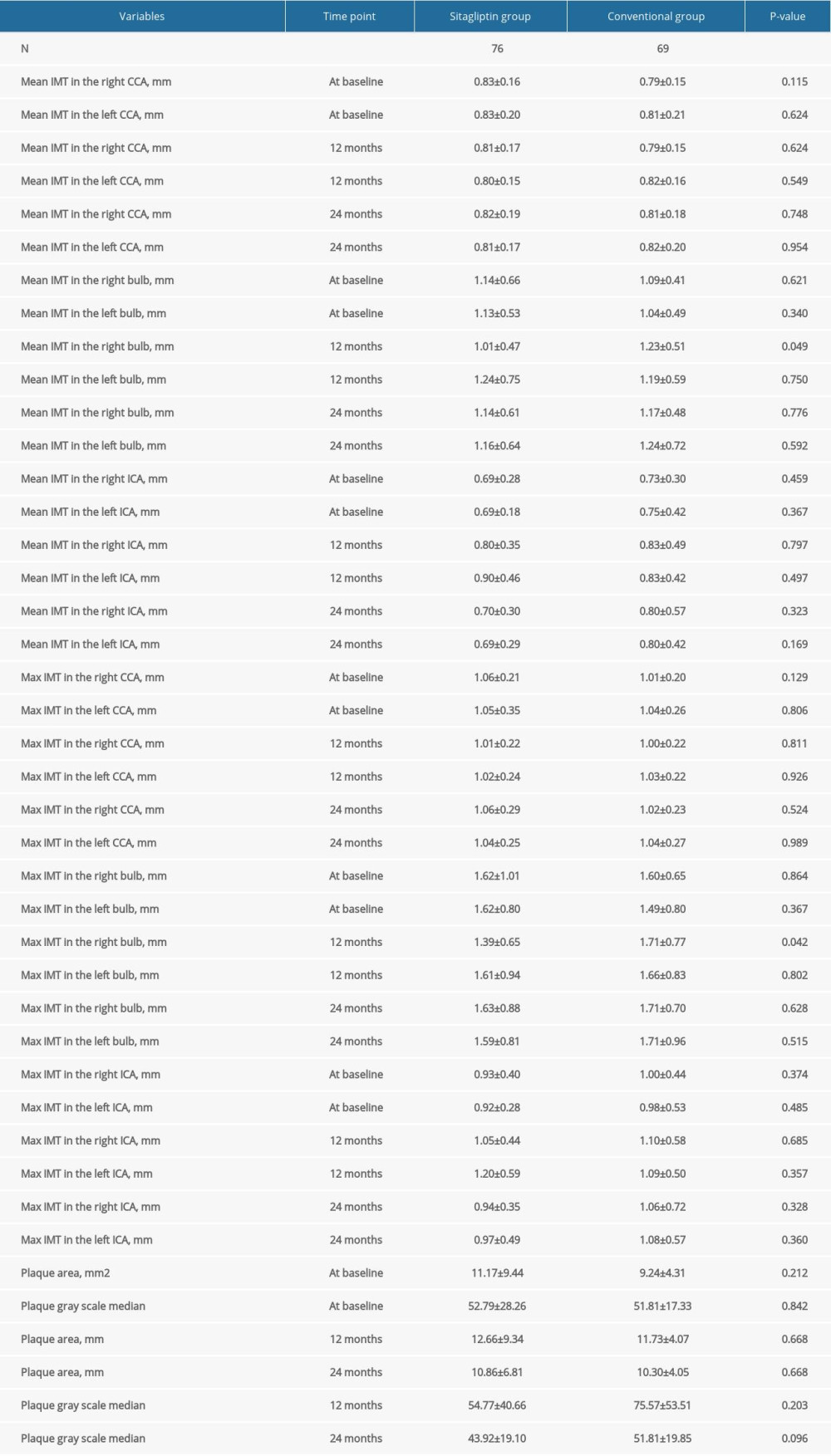 Table 5. Comparison of blood lipid, blood glucose, and other indexes in male participants at 24 months.
Table 5. Comparison of blood lipid, blood glucose, and other indexes in male participants at 24 months.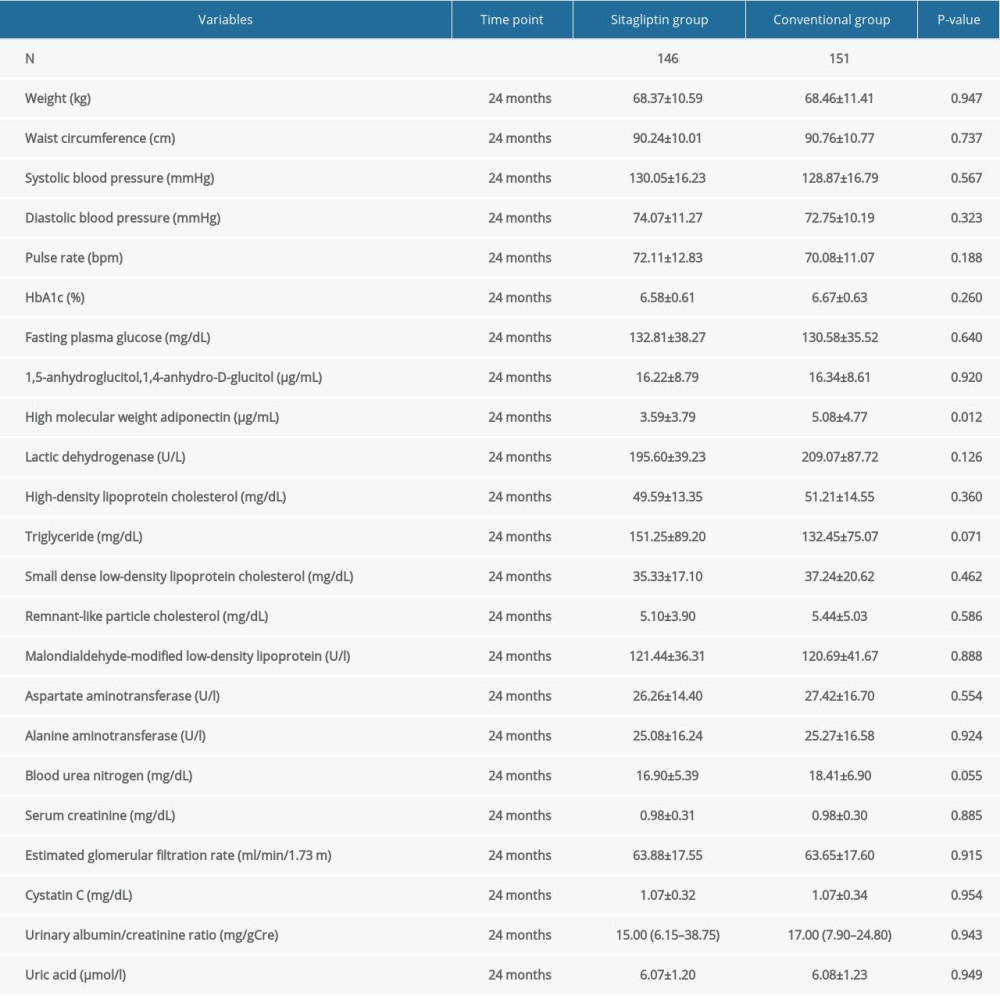 Table 6. Comparison of blood lipid, blood glucose, and other indexes in female participants at 24 months.
Table 6. Comparison of blood lipid, blood glucose, and other indexes in female participants at 24 months.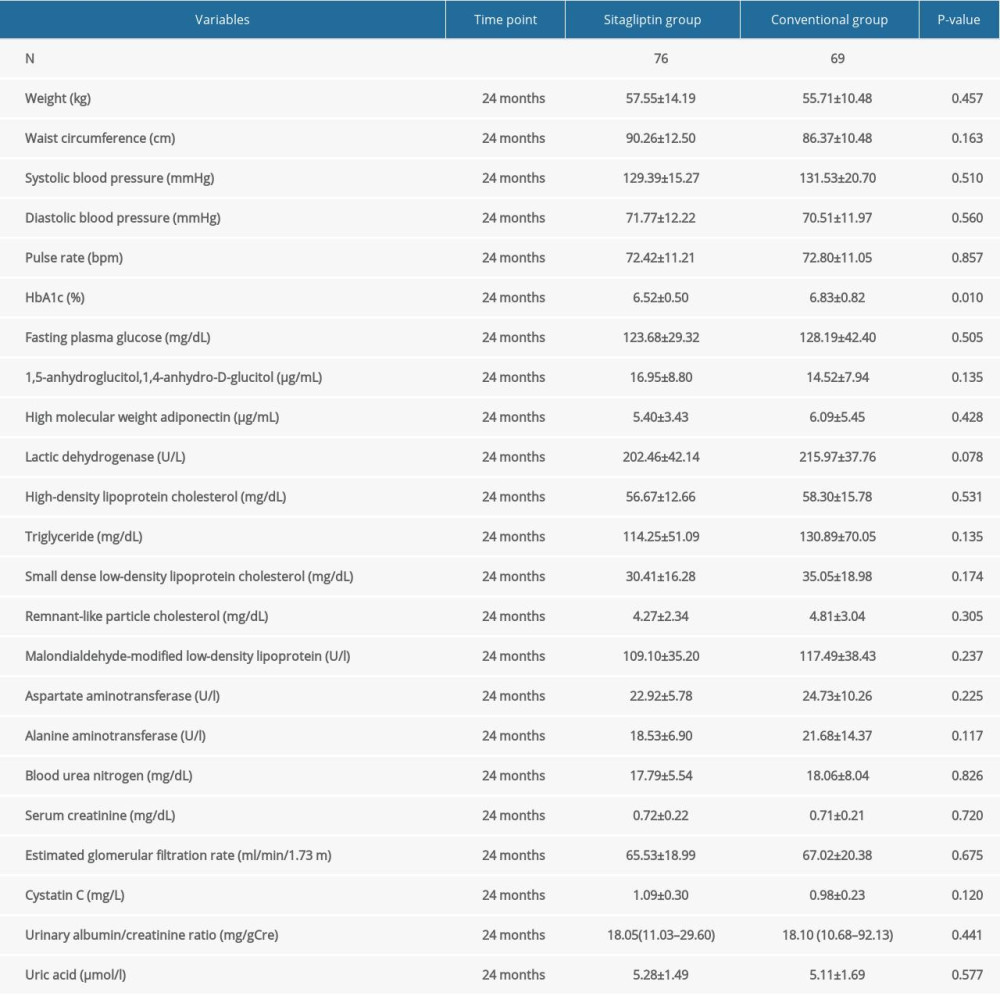
References
1. Wang J, Sun C, Gerdes N, Interleukin 18 function in atherosclerosis is mediated by the interleukin 18 receptor and the Na-Cl co-transporter: Nat Med, 2015; 21(7); 820-26
2. Robbins CS, Hilgendorf I, Weber GF, Local proliferation dominates lesional macrophage accumulation in atherosclerosis: Nat Med, 2013; 19(9); 1166-72
3. Liebeskind DS, Cotsonis GA, Saver JLWarfarin-Aspirin Symptomatic Intracranial Disease (WASID) Investigators, Collaterals dramatically alter stroke risk in intracranial atherosclerosis: Ann Neurol, 2011; 69(6); 963-74
4. Dadu RT, Fornage M, Virani SS, Cardiovascular biomarkers and subclinical brain disease in the atherosclerosis risk in communities study: Stroke, 2013; 44(7); 1803-8
5. Ogagarue ER, Lutsey PL, Klein R, Association of ideal cardiovascular health metrics and retinal microvascular findings: The Atherosclerosis Risk in Communities Study: J Am Heart Assoc, 2013; 2(6); e000430
6. Muscogiuri G, Annweiler C, Duval G, Vitamin D and cardiovascular disease: From atherosclerosis to myocardial infarction and stroke: Int J Cardiol, 2017; 230; 577-84
7. Becattini C, Dentali F, Camporese G, Carotid atherosclerosis and risk for ischemic stroke in patients with atrial fibrillation on oral anticoagulant treatment: Atherosclerosis, 2018; 271; 177-81
8. Lv P, Jin H, Liu Y, Comparison of risk factor between lacunar stroke and large artery atherosclerosis stroke: A cross-sectional study in China: PLoS One, 2016; 11(3); e0149605
9. Yin JH, Song ZY, Shan PF, Age- and gender-specific prevalence of carotid atherosclerosis and its association with metabolic syndrome in Hangzhou, China: Clin Endocrinol (Oxf), 2012; 76(6); 802-9
10. Amer MS, Khater MS, Omar OH, Association between Framingham risk score and subclinical atherosclerosis among elderly with both type 2 diabetes mellitus and healthy subjects: Am J Cardiovasc Dis, 2014; 4(1); 14-19
11. Yahagi K, Kolodgie FD, Lutter C, Pathology of human coronary and carotid artery atherosclerosis and vascular calcification in diabetes mellitus: Arterioscler Thromb Vasc Biol, 2017; 37(2); 191-204
12. Nozue T, Yamamoto S, Tohyama STRUTH Investigators, Impact of diabetes mellitus on coronary atherosclerosis and plaque composition under statin therapy – subanalysis of the TRUTH study: Circ J, 2012; 76(9); 2188-96
13. Svensson AK, Svensson T, Kitlinski M, Incident diabetes mellitus may explain the association between sleep duration and incident coronary heart disease: Diabetologia, 2018; 61(2); 331-41
14. Doi Y, Ninomiya T, Hata J, Impact of glucose tolerance status on development of ischemic stroke and coronary heart disease in a general Japanese population: The Hisayama study: Stroke, 2010; 41(2); 203-9
15. Overbeek JA, Bakker M, van der Heijden AAWA, Risk of dipeptidyl peptidase-4 (DPP-4) inhibitors on site-specific cancer: A systematic review and meta-analysis: Diabetes Metab Res Rev, 2018; 34(5); e3004
16. Patel BD, Ghate MD, Recent approaches to medicinal chemistry and therapeutic potential of dipeptidyl peptidase-4 (DPP-4) inhibitors: Eur J Med Chem, 2014; 74; 574-605
17. Matsubara J, Sugiyama S, Sugamura K, A dipeptidyl peptidase-4 inhibitor, des-fluoro-sitagliptin, improves endothelial function and reduces atherosclerotic lesion formation in apolipoprotein E-deficient mice: J Am Coll Cardiol, 2012; 59(3); 265-76
18. Zeng Y, Li C, Guan M, The DPP-4 inhibitor sitagliptin attenuates the progress of atherosclerosis in apolipoprotein-E-knockout mice via AMPK- and MAPK-dependent mechanisms: Cardiovasc Diabetol, 2014; 13; 32
19. Oyama J, Murohara T, Kitakaze MPROLOGUE Study Investigators, The effect of sitagliptin on carotid artery atherosclerosis in type 2 diabetes: The PROLOGUE randomized controlled trial: PLoS Med, 2016; 13(6); e1002051
20. Nozue T, Fukui K, Koyama YTRUST Investigators, Effects of sitagliptin on coronary atherosclerosis in patients with type 2 diabetes-A serial integrated backscatter-intravascular ultrasound study: Am J Cardiovasc Dis, 2016; 6(4); 153-62
21. Green JB, Bethel MA, Armstrong PWTECOS Study Group, Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes: N Engl J Med, 2015; 373(3); 232-42 [Erratum in: N Engl J Med. 2015;373(6):586]
22. McGuire DK, Van de Werf F, Armstrong PWTrial Evaluating Cardiovascular Outcomes With Sitagliptin (TECOS) Study Group, Association between sitagliptin use and heart failure hospitalization and related outcomes in type 2 diabetes mellitus: Secondary analysis of a randomized clinical trial: JAMA Cardiol, 2016; 1(2); 126-35
23. Katakami N, Mita T, Irie YSitagliptin Preventive study of Intima-media thickness Evaluation (SPIKE) Collaborators, Effect of sitagliptin on tissue characteristics of the carotid wall in patients with type 2 diabetes: A post hoc sub-analysis of the sitagliptin preventive study of intima-media thickness evaluation (SPIKE): Cardiovasc Diabetol, 2018; 17(1); 24
24. Fan M, Li Y, Zhang S, Effects of sitagliptin on lipid profiles in patients with type 2 diabetes mellitus: A meta-analysis of randomized clinical trials: Medicine (Baltimore), 2016; 95(2); e2386
25. Oyama J, Ishizu T, Sato Y, Kodama K, Rationale and design of a study to evaluate the effects of sitagliptin on atherosclerosis in patients with diabetes mellitus: PROLOGUE study: Int J Cardiol, 2014; 174(2); 383-84
26. Plasencia Martínez JM, Garcia Santos JM, Paredes Martinez ML, Pastor AM, Carotid intima-media thickness and hemodynamic parameters: Reproducibility of manual measurements with Doppler ultrasound: Med Ultrason, 2015; 17(2); 167-74
27. Giannarelli C, Bianchini E, Bruno RM, Local carotid stiffness and intima-media thickness assessment by a novel ultrasound-based system in essential hypertension: Atherosclerosis, 2012; 223(2); 372-77
28. Jung JM, Kwon DY, Han C, Increased carotid intima-media thickness and plasma homocysteine levels predict cardiovascular and all-cause death: A population-based cohort study: Eur Neurol, 2013; 70(1–2); 1-5
29. Zhang Y, Fang X, Hua Y, Carotid artery plaques, carotid intima-media thickness, and risk of cardiovascular events and all-cause death in older adults: A 5-year prospective, community-based study: Angiology, 2018; 69(2); 120-29
30. Greenland P, Alpert JS, Beller GAAmerican College of Cardiology Foundation; American Heart Association, 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: J Am Coll Cardiol, 2010; 56(25); e50-103
31. Shimoda S, Iwashita S, Ichimori S, Efficacy and safety of sitagliptin as add-on therapy on glycemic control and blood glucose fluctuation in Japanese type 2 diabetes subjects ongoing with multiple daily insulin injections therapy: Endocr J, 2013; 60(10); 1207-14
32. Maruhashi T, Higashi Y, Kihara YPROLOGUE Study Investigators, Long-term effect of sitagliptin on endothelial function in type 2 diabetes: A sub-analysis of the PROLOGUE study: Cardiovasc Diabetol, 2016; 15(1); 134
33. Kubota Y, Miyamoto M, Takagi G, The dipeptidyl peptidase-4 inhibitor sitagliptin improves vascular endothelial function in type 2 diabetes: J Korean Med Sci, 2012; 27(11); 1364-70
34. Selwaness M, van den Bouwhuijsen Q, van Onkelen RS, Atherosclerotic plaque in the left carotid artery is more vulnerable than in the right: Stroke, 2014; 45(11); 3226-30
35. Katranas SA, Kelekis AL, Antoniadis AP, Differences in stress forces and geometry between left and right coronary artery: A pathophysiological aspect of atherosclerosis heterogeneity: Hellenic J Cardiol, 2015; 56(3); 217-23
36. Yanai H, Adachi H, Hamasaki H, Effects of 6-month sitagliptin treatment on glucose and lipid metabolism, blood pressure, body weight and renal function in type 2 diabetic patients: A chart-based analysis: J Clin Med Res, 2012; 4(4); 251-58
37. Kawasaki I, Hiura Y, Tamai A, Sitagliptin reduces the urine albumin-to-creatinine ratio in type 2 diabetes through decreasing both blood pressure and estimated glomerular filtration rate: J Diabetes, 2015; 7(1); 41-46
Figures
 Figure 1. Male patients. (A) Comparison of mean IMT in the right ICA between the 2 groups at 12 months. (B) Comparison of maximum IMT in the right ICA between the 2 groups at 12 months. (C) Comparison of maximum IMT in the right ICA between the 2 groups at 24 months.
Figure 1. Male patients. (A) Comparison of mean IMT in the right ICA between the 2 groups at 12 months. (B) Comparison of maximum IMT in the right ICA between the 2 groups at 12 months. (C) Comparison of maximum IMT in the right ICA between the 2 groups at 24 months. Figure 2. Female patients. (A) Comparison of mean IMT in the right bulb between the 2 groups at 12 months. Figure (B) Comparison of maximum IMT in the right bulb between the 2 groups at 12 months.
Figure 2. Female patients. (A) Comparison of mean IMT in the right bulb between the 2 groups at 12 months. Figure (B) Comparison of maximum IMT in the right bulb between the 2 groups at 12 months. Tables
 Table 1. Baseline characteristics of male participants.
Table 1. Baseline characteristics of male participants. Table 2. Baseline characteristics of female participants.
Table 2. Baseline characteristics of female participants. Table 3. Comparison of atherosclerotic parameters at baseline, 12 months, and 24 months in male participants.
Table 3. Comparison of atherosclerotic parameters at baseline, 12 months, and 24 months in male participants. Table 4. Comparison of atherosclerotic parameters at baseline, 12 months, and 24 months in female participants.
Table 4. Comparison of atherosclerotic parameters at baseline, 12 months, and 24 months in female participants. Table 5. Comparison of blood lipid, blood glucose, and other indexes in male participants at 24 months.
Table 5. Comparison of blood lipid, blood glucose, and other indexes in male participants at 24 months. Table 6. Comparison of blood lipid, blood glucose, and other indexes in female participants at 24 months.
Table 6. Comparison of blood lipid, blood glucose, and other indexes in female participants at 24 months. Table 1. Baseline characteristics of male participants.
Table 1. Baseline characteristics of male participants. Table 2. Baseline characteristics of female participants.
Table 2. Baseline characteristics of female participants. Table 3. Comparison of atherosclerotic parameters at baseline, 12 months, and 24 months in male participants.
Table 3. Comparison of atherosclerotic parameters at baseline, 12 months, and 24 months in male participants. Table 4. Comparison of atherosclerotic parameters at baseline, 12 months, and 24 months in female participants.
Table 4. Comparison of atherosclerotic parameters at baseline, 12 months, and 24 months in female participants. Table 5. Comparison of blood lipid, blood glucose, and other indexes in male participants at 24 months.
Table 5. Comparison of blood lipid, blood glucose, and other indexes in male participants at 24 months. Table 6. Comparison of blood lipid, blood glucose, and other indexes in female participants at 24 months.
Table 6. Comparison of blood lipid, blood glucose, and other indexes in female participants at 24 months. In Press
06 Mar 2024 : Clinical Research
Prevalence and Variation of Medical Comorbidities in Oral Surgery Patients: A Retrospective Study at Jazan ...Med Sci Monit In Press; DOI: 10.12659/MSM.943884
08 Mar 2024 : Clinical Research
Evaluation of Foot Structure in Preschool Children Based on Body MassMed Sci Monit In Press; DOI: 10.12659/MSM.943765
15 Apr 2024 : Laboratory Research
The Role of Copper-Induced M2 Macrophage Polarization in Protecting Cartilage Matrix in OsteoarthritisMed Sci Monit In Press; DOI: 10.12659/MSM.943738
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








