10 April 2024: Clinical Research
Comparison of Remimazolam and Propofol on Postoperative Delirium in Elderly Patients Undergoing Radical Resection of Colon Cancer: A Single-Center Prospective Randomized Controlled Study
Tianlin Liu1ABCDEFG*, Haochen Zhao1BEG, Xiaochen Zhao1BCF, Min Qu1ABCDDOI: 10.12659/MSM.943784
Med Sci Monit 2024; 30:e943784
Abstract
BACKGROUND: We compared the effect of remimazolam and propofol intravenous anesthesia on postoperative delirium in elderly patients undergoing laparoscopic radical resection of colon cancer.
MATERIAL AND METHODS: One hundred patients undergoing elective radical operation of colon cancer under general anesthesia were divided into a remimazolam group (group R) and propofol group (group P) by a random number table method. During anesthesia induction and maintenance, group R was intravenously injected with remimazolam to exert sedation; however, in group P, propofol was injected instead of remimazolam. The occurrence of postoperative delirium was assessed with the Confusion Assessment Method for the Intensive Care Unit scale and postoperative pain was assessed with the visual analogue score (VAS). The primary outcome measures were the incidence and duration of delirium within 7 days following surgery. Secondary outcome measures included postoperative VAS scores, intraoperative anesthetic drug dosage, and adverse reactions, including nausea and vomiting, hypoxemia, and respiratory depression.
RESULTS: There was no significant difference in baseline data between the 2 groups (P>0.05). There was no statistically significant difference in the incidence and duration of postoperative delirium between the 2 groups (P>0.05). There were no significant differences in VAS scores, remifentanil consumption, and adverse reactions, including nausea and vomiting, hypoxemia, and respiratory depression between the 2 groups (P>0.05).
CONCLUSIONS: In elderly patients undergoing radical colon cancer surgery, remimazolam administration did not improve or aggravate the incidence and duration of delirium, compared with propofol.
Keywords: Aged, Colonic Neoplasms, Delirium, Propofol, Remimazolam
Introduction
Postoperative delirium is one of the common postoperative complications in elderly patients, which is characterized by disturbance of consciousness, hallucination, inattention, insomnia, and other symptoms [1]. The incidence of postoperative delirium was 13.8% to 30% in elderly patients undergoing radical colonic cancer surgery [2]. It affected the rapid recovery of patients, was associated with higher postoperative complications and mortality, and increased the difficulty of nursing and the socio-economic burden on families [3]. The mechanism of postoperative delirium remains complex and has not been determined to date. However, higher age, intraoperative opioid use, intraoperative hypotension, intraoperative benzodiazepine use, frailty, and postoperative pain are all traditionally reported risk factors for postoperative delirium [4–9].
Propofol and midazolam are the most commonly used anesthetic agents used to induce and maintain sedation during general anesthesia. Propofol is often associated with injection pain [10], respiratory depression [11], and circulatory depression [12]. Midazolam is a benzodiazepine drug that acts on GABA receptors to exert sedative and hypnotic effects, but its metabolites have been found to have certain activity in clinical application, which can prolong the recovery time of patients. Therefore, there is need for a sedative drug that is fast acting and has fast metabolism, little interference to the respiratory system and circulatory systems, and no accumulation of metabolites.
Remimazolam, a novel ultra-short-acting benzodiazepine drug, was obtained by introducing a metabolizable methyl propionate side chain into the midazolam benzodiazepine parent ring structure [13]. Previous clinical studies have found that the peak time of blood concentration of remimazolam is about 3 min, which is shorter than that of midazolam and longer than that of propofol [14]. The average retention time of remimazolam is shorter than that of midazolam [15]. The pharmacokinetics of remimazolam are linear, the clearance is independent of body mass, and the clearance rate is higher than that of midazolam [16]. Remimazolam can be rapidly hydrolyzed by nonspecific esterase in blood to metabolize zolam propionic acid, without pharmacological activity. The affinity of remimazolam metabolites to GABA receptors is only 1/400 that of midazolam [17]. Therefore, remimazolam is independent of cellular P450 enzyme and liver metabolism and has rapid efficacy, short elimination half-life, short maintenance time, and no accumulation. With the specific antagonist flumazenil, the anesthetic effect can be reversed quickly, and the time-dose-dependent half-life is not affected by the infusion time. Long-term or high-dose administration will not cause drug accumulation. Results from a recent prospectively randomized controlled trial suggested that remimazolam can be safely used under general anesthesia in patients undergoing urological surgery, compared with propofol, and the hemodynamics were more stable; however, it suggested that remimazolam infusion resulted in more cases of postoperative delirium than did propofol infusion, although the difference was not statistically significant [18]. However, it was suggested in another clinical trial that remimazolam improves postoperative cognitive function in patients undergoing hip replacement, compared with propofol [19].
Overall, the effect of remimazolam on postoperative delirium has been controversial to date.
In addition, the effect of remimazolam on postoperative delirium in patients undergoing radical colon cancer surgery has not been reported. Therefore, this study hypothesized that remimazolam could improve postoperative delirium in elderly patients undergoing radical colon cancer surgery, compared with propofol, and investigated the occurrence of other adverse reactions.
Material and Methods
PATIENTS:
The single-center prospective randomized controlled study lasted over 3 months, from October 8, 2023, to January 10, 2024. A total of 100 patients were included in the study.
All plans were approved by the Ethics Committee of Cangzhou Central Hospital (2023-159-02). This study was pre-registered in the Chinese Clinical Trial Center (ChiCTR2300076384) and conducted in accordance with the Declaration of Helsinki and CONSORT guidelines. Written informed consent was obtained from each patient in advance.
The inclusion criteria were as follows: (1) patients undergoing elective radical resection of colon cancer surgery; (2) elderly patients aged ≥65 years; and (3) American Society of Anesthesiologists (ASA) Grade I–III. The exclusion criteria were as follows: (1) patients with a history of severe mental illness or pre-existing central nervous system diseases; (2) a recent history of alcohol abuse; (3) severe visual, hearing, or speech impairments and poor communication skills; (4) allergy to the study drug; and (5) severe heart and lung disease, or liver or kidney failure.
RANDOMIZATION AND BLINDING:
One researcher who was not involved in this study completed the random numbers with an online tool (
ANESTHESIA METHOD:
Before entering the operating room, all patients fasted for 8 h and did not drink for 4 h. Electrocardiograph, heart rate, blood pressure, oxygen saturation (SPO2), and partial pressure of end-expiratory carbon dioxide (PETCO2) were monitored after entering the operating room. Meanwhile, peripheral venous access was established for infusion, and adequate oxygen was given.
ANESTHESIA INDUCTION:
The anesthesia induction scheme was as follows: In group R, patients were given intravenously in sequence with sufentanil (Yichang Renfu Pharmaceutical Co., Ltd., H20054171) 0.1 to 2 μg/kg+remimazolam besylate (Jiangsu Hengrui Pharmaceutical Co., Ltd., H20190034) 0.1 to 0.2 mg/kg+cisatracurium (Shanghai Hengrui Pharmaceutical Co., Ltd., H20060869) 0.2 mg/kg. Patients in Group P were intravenously injected with sufentanil (Yichang Renfu Pharmaceutical Co., Ltd., H20054171) 0.1 to 2 μg/kg+propofol (Beijing Fresenius Kabi Pharmaceutical Co., Ltd., J20160041) 1 to 2 mg/kg+cisatracurium (Shanghai Hengrui Pharmaceutical Co., Ltd., H20060869) 0.2 mg/kg. After the drug took effect, endotracheal intubation was performed under visual laryngoscope, mechanical ventilation was performed in both groups, and the tidal volume was set at 8 to 10 mL/kg. The ratio of aspiration to breath was 1: 2, oxygen flow was 2 L/min, the respiratory rate was 10 to 12 times/min.
ANESTHESIA MAINTENANCE:
Both groups received infusions of remifentanil, propofol, or remimazolam besylate with a TCI pump (Yichang Humanwell Pharmaceutical Co., Ltd., China). The anesthesia maintenance regimen was as follows: Patients in group R were intravenously injected with remimazolam besylate (Jiangsu Hengrui Pharmaceutical Co., Ltd., H20190034) 0.4 to 1.2 mg/(kg·h)+remifentanil (Yichang Renfu Pharmaceutical Co., Ltd., H20030197) 0.1 to 0.2μg/(kg·min); Patients in group P were intravenously injected with propofol (Beijing Fresenius Kabi Pharmaceutical Co., Ltd., J20160041) 4 to 10 mg/(kg·h)+remifentanil (Yichang Renfu Pharmaceutical Co., Ltd., H20030197) 0.1 to 0.2 μg/(kg·min). In both groups, cisatracurium (Shanghai Hengrui Pharmaceutical Co., Ltd., H20060869) 0.1 to 0.2 mg/kg was added to maintain muscle relaxation intermittently. The pumping rate of remimazolam besylate, propofol, and remifentanil was adjusted according to the bispectral index (BIS) value to maintain an appropriately depth of anesthesia, with a BIS value of 40 to 60. During the operation, end-respiratory PCO2 was maintained at 35 to 45 mm Hg. Flurbiprofen axel 50 mg and toanisetron 5 mg were given 30 min before the end of surgery. Five minutes before the end of surgery, all infusions of anesthetic drugs were stopped. After surgery, the tracheal catheter was removed when the patient regained consciousness, the tidal volume had returned to 8 mL/kg, and all hemodynamic parameters (including heart rate and blood pressure) had returned to the preoperative baseline level.
In addition, vasoactive agents were administered intraoperatively at the discretion of the anesthesiologist, based on the patient’s hemodynamic indicators. For example, norepinephrine was given when the systolic blood pressure was 40 mm Hg below the baseline level, urapidil was given when it was 20% above the baseline value, and atropine was given intravenously when the heart rate was below 50 beats/min.
POSTOPERATIVE ANALGESIA:
All patients received a postoperative analgesic pump. The formula for the pain pump was 1.5 μg/kg sufentanil mixed with 100 mL of normal saline. To observe postoperative sufentanil dosage, we abandoned the patient control function in the analgesic pump. The flow rate of the analgesic pump was 2 mL/h. If the patient’s visual analogue score (VAS) was greater than or equal to 4 points, 50 mg intravenous injection of flurbiprofen axidate was given.
MEASUREMENT INDEX:
The incidence and duration of delirium within 7 days after surgery were recorded in both groups. The Confusion Assessment Method for the Intensive Care Unit scale (CAM-ICU) and the Richmond Agitation-Sedation Scale (RASS) were used to evaluate the occurrence of postoperative delirium and level of consciousness [20]. First, the patient’s sedation was assessed using the RASS Scale, with a RASS score of −4 or −5 indicating that the patient was unconscious and should be reassessed later. If the RASS score was ≥-3, the CAM-ICU assessment was continued. Delirium was evaluated twice a day, once from 8 AM to 10 AM and once from 6 PM to 8 PM [21]. The CAM-ICU scale contains the following aspects: (1) acute onset and course fluctuation of consciousness; (2) attention deficit; (3) altered level of consciousness; and (4) confusion. The diagnosis of postoperative delirium must meet (1)(2)(3) or (1)(2)(4).
The occurrence of adverse reactions, including nausea and vomiting, respiratory depression and hypoxemia, were recorded in the 2 groups. Nausea and vomiting were subjective symptoms, which referred to postoperative gastrointestinal discomfort.
The criteria for respiratory depression was a respiratory rate ≤10 times/min. Hypoxemia referred to intraoperative SpO2 ≤95%.
STATISTICAL METHODS:
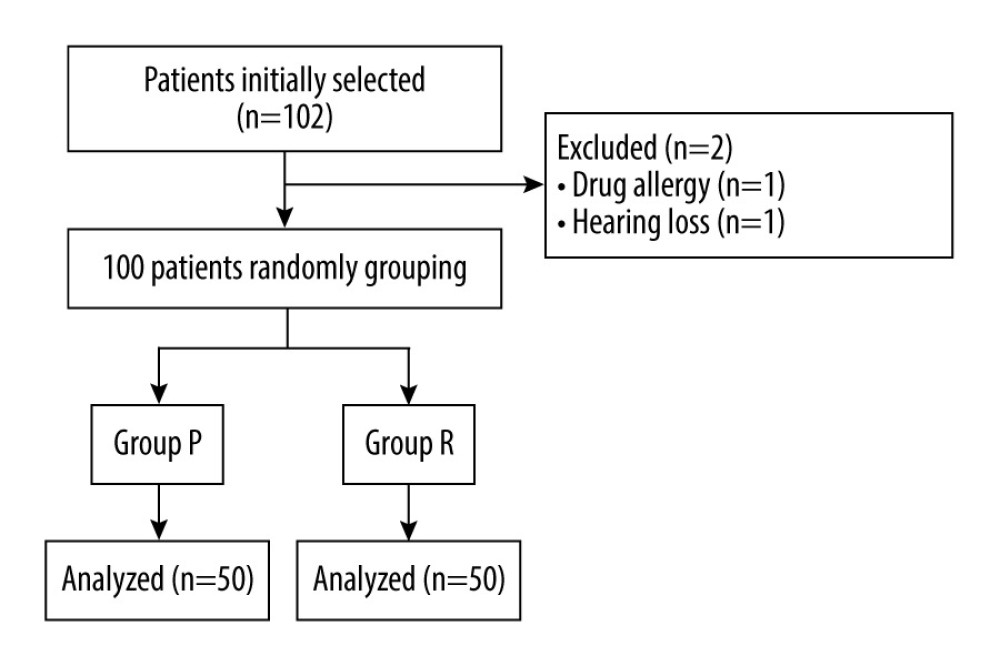
Sample size was calculated using a web calculator (http://powerandsamplesize.com/Calculators/). It has been reported that the incidence of delirium after radical resection of colon cancer is 24% [22]. The sample size was 1: 1, α=0.05, β=0.2, and the minimum sample size was estimated to be 43 cases in each group. Considering the shedding rate, 102 patients were finally included.
SPSS Statistics 21 software was used for statistical analysis. The measurement data were expressed as mean±standard deviation or median±quartile according to normal distribution. The independent sample
Results
COMPARISON OF GENERAL DATA BETWEEN THE 2 GROUPS:
A total of 102 patients were included in this study, of which 2 patients were excluded: one due to drug allergy, and the other for inability to follow up due to hearing loss. The remaining 100 patients were randomly divided into group R and group P (Figure 1).
There was no significant difference in sex, age, weight, body mass index, ASA classification, years of education, hypertension, diabetes, coronary heart disease, smoking, and alcohol consumption between the 2 groups (P>0.05), as shown in Table 1.
COMPARISON OF INTRAOPERATIVE AND POSTOPERATIVE INDEXES BETWEEN THE 2 GROUPS:
There was no significant difference in surgical duration, anesthesia time, infusion volume, urine output, blood loss, remifentanil consumption,VAS scores, and BIS values between the 2 groups (P>0.05, Table 2).
COMPARISON OF INCIDENCE AND DURATION OF POSTOPERATIVE DELIRIUM BETWEEN THE 2 GROUPS:
Among the 100 patients selected, 26 patients developed postoperative delirium, including 15 patients in group P and 11 patients in group R. There was no statistical difference in the incidence of postoperative delirium between the 2 groups (P=0.362; Table 3).
The cumulative duration of postoperative delirium was also used to assess the effect of remimazolam on postoperative delirium. In group P, postoperative delirium occurred on a total of 28 days; however, it occurred 23 days in group R. There was no significant difference in the duration of accumulation between the 2 groups (P=0.317; Table 3).
COMPARISON OF OTHER ADVERSE REACTIONS BETWEEN THE 2 GROUPS:
There was no significant difference in the incidence of nausea and vomiting, hypoxemia, and respiratory depression between the 2 groups (P>0.05; Table 4).
Discussion
In this study, we found that in elderly patients undergoing radical colon cancer surgery, the incidence and cumulative duration of postoperative delirium were not significantly different between the remimazolam and the propofol groups. In addition, there were no statistically significant differences in the incidence of nausea and vomiting, hypoxemia, and respiratory depression between the 2 groups.
Postoperative delirium is one of the common complications in patients with dementia undergoing radical resection for colon cancer [2]. Patients undergoing radical colon cancer surgery are at a high risk of postoperative delirium due to significant trauma and a long operation time. Due to the degenerative changes of various organs in the body of elderly patients and underlying diseases, such as hypertension, diabetes, and coronary heart disease, the body reserve capacity is poor and unable to provide the maximum additional safety requirements for surgery, trauma, and rehabilitation. Studies have shown that older patients are more likely to develop postoperative delirium than are younger patients [23,24].
In this study, we reported that the incidence of postoperative delirium in elderly patients undergoing radical colon cancer resection was 26%, which was consistent with previous studies [25]. Traditional evidence suggested that risk factors for postoperative delirium include advanced age, the use of opioids and benzodiazepines, postoperative pain, and the stress response from surgery [26–30]. A previous report [30] has suggested that benzodiazepines increase the risk of postoperative delirium. The reason may be due to the study’s use of lorazepam, which has a long half-life of 15 h and is metabolized through the liver and kidney. Marcantonio et al found that long-acting benzodiazepines are more likely to cause postoperative delirium than are short-acting benzodiazepines [31]. However, a recent review noted that benzodiazepines did not increase the risk of postoperative delirium [32]. Therefore, we investigated the effect of a new benzodiazepine drug remimazolam on postoperative delirium in elderly patients undergoing laparoscopic radical resection of colon cancer. Our results suggested that anesthesia induction and maintenance of remimazolam did not increase the incidence and cumulative duration of postoperative delirium, compared with propofol; the result was consistent with that of the previous study [32]. The reason may be that, compared with other benzodiazepines, remimazolam does not undergo liver and kidney metabolism, the metabolites have no pharmacological activity, and there is no accumulation after long-term infusion.
In addition, remimazolam has been reported to reduce cortisol levels, compared with propofol [18], and elevated cortisol levels can have adverse effects on cognition [33]. This can be another reason remimazolam did not increase the incidence and cumulative duration of postoperative delirium.
As an intravenous anesthetic commonly used in the clinic, propofol has the characteristics of rapid induction and recovery, but it also has some adverse reactions, such as respiration and circulation inhibition, injection pain, metabolic acidosis, and propofol infusion syndrome.
A meta-analysis [34] involving 1813 patients from 10 studies showed that the incidence of hypotension, injection pain, and hypoxemia in the remimazolam group was lower than that in the propofol group. A randomized controlled study comparing the effects of propofol and remimazolam on hysteroscopy showed that there were fewer cases of nausea, vomiting, respiratory depression, hypoxemia, and injection pain in the remimazolam group than in the propofol group [35]. The results of these studies confirmed the results of the above experiments.
Our research still had some limitations. First, this was a single-center study with a relatively small sample size; therefore, future multi-center studies with a large sample size should be conducted. Second, in this study, only the CAM-ICU scale was used to evaluate the occurrence of postoperative delirium. In the future, 2 or more evaluation scales can be used to avoid subjective bias. Third, although this study was a double-blind randomized controlled study, in principle, the anesthesiologists did not know the grouping of patients, but the color of propofol and remimazolam were very different; therefore, even with the use of a brown syringe and extension tube, it was still possible to identify the different drugs, which may have affected the results. Finally, our study recorded various clinical indicators without further studies on the molecular mechanism. In future studies, blood samples can be collected to detect cortisol, inflammatory factors, and other indicators to further explore the relationship between remimazolam and postoperative delirium.
Conclusions
Compared with propofol, remimazolam did not increase the incidence of postoperative delirium, nor did it prolong the cumulative duration of postoperative delirium in elderly patients undergoing radical resection of colon cancer.
Tables
Table 1. Demographic characteristics of patients in both groups.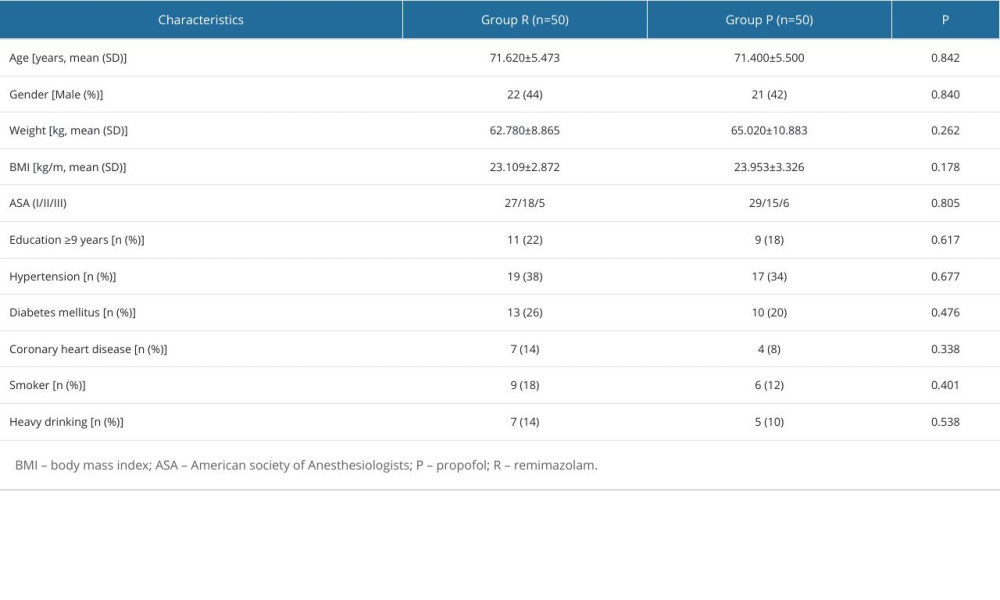 Table 2. Comparison of intraoperative and postoperative indexes between the 2 groups.
Table 2. Comparison of intraoperative and postoperative indexes between the 2 groups.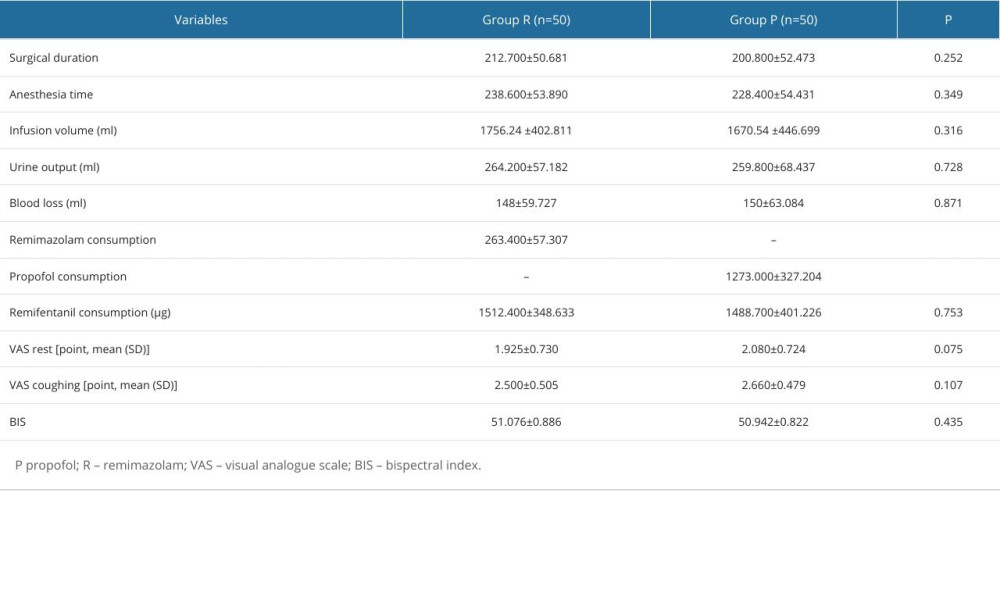 Table 3. Comparison of incidence and cumulative duration of postoperative delirium between the 2 groups [n (%)].
Table 3. Comparison of incidence and cumulative duration of postoperative delirium between the 2 groups [n (%)].![Comparison of incidence and cumulative duration of postoperative delirium between the 2 groups [n (%)].](https://jours.isi-science.com/imageXml.php?i=t3-medscimonit-30-e943784.jpg&idArt=943784&w=1000) Table 4. The occurrence of adverse reactions in the 2 groups [n (%)].
Table 4. The occurrence of adverse reactions in the 2 groups [n (%)].![The occurrence of adverse reactions in the 2 groups [n (%)].](https://jours.isi-science.com/imageXml.php?i=t4-medscimonit-30-e943784.jpg&idArt=943784&w=1000)
References
1. Lee YL, Wong J, Ng SY, Delirium in patients following general anaesthesia: Ann Acad Med Singap, 2022; 51(2); 71-73
2. Nishizawa Y, Hata T, Takemasa I, Clinical benefits of single-incision laparoscopic surgery for postoperative delirium in elderly colon cancer patients: Surg Endosc, 2018; 32(3); 1434-40
3. O’Hanlon S, Baxter M, Hosie A, Postoperative delirium in older patients with cancer: The role of psychological distress and social support: Curr Opin Support Palliat Care, 2022; 16(1); 38-47
4. Bramley P, McArthur K, Blayney A, McCullagh I, Risk factors for postoperative delirium: An umbrella review of systematic reviews: Int J Surg, 2021; 93; 106063
5. Wachtendorf LJ, Azimaraghi O, Santer P, Association between intraoperative arterial hypotension and postoperative delirium after noncardiac surgery: A retrospective multicenter cohort study: Anesth Analg, 2022; 134(4); 822-33
6. Buren MA, Theologis A, Zuraek A, Lidocaine Infusion for the Management of Postoperative Pain and Delirium (LIMPP): Protocol for a randomised control trial: BMJ Open, 2022; 12(6); e059416
7. Gold C, Ray E, Christianson D, Risk factors for delirium in elderly patients after lumbar spinal fusion: Clin Neurol Neurosurg, 2022; 219; 107318
8. Swarbrick CJ, Partridge JSL, Evidence-based strategies to reduce the incidence of postoperative delirium: A narrative review: Anaesthesia, 2022; 77(Suppl 1); 92-101
9. Zhang XM, Jiao J, Xie XH, Wu XJ, The association between frailty and delirium among hospitalized patients: an updated meta-analysis: J Am Med Dir Assoc, 2021; 22(3); 527-34
10. Xu C, Wei X, Zhang C, Esketamine prevents propofol-induced injection pain: Randomized controlled trial: Front Pharmacol, 2022; 13; 991559
11. Jiang J, Jiao Y, Gao P, Propofol differentially induces unconsciousness and respiratory depression through distinct interactions between GABAA receptor and GABAergic neuron in corresponding nuclei: Acta Biochim Biophys Sin (Shanghai), 2021; 53(8); 1076-87
12. Qi XR, Sun JY, An LX, Zhang K, Effect of intravenous lidocaine on the ED50 of propofol for inserting gastroscope without body movement in adult patients: A randomized, controlled study: BMC Anesthesiol, 2022; 22(1); 319
13. Chae D, Kim HC, Song Y, Pharmacodynamic analysis of intravenous bolus remimazolam for loss of consciousness in patients undergoing general anaesthesia: A randomised, prospective, double-blind study: Br J Anaesth, 2022; 129(1); 49-57 [Erratum in: Br J Anaesth. 2022;129(5):831]
14. Schüttler J, Eisenried A, Lerch M, Pharmacokinetics and pharmacodynamics of remimazolam (CNS 7056) after continuous infusion in healthy male volunteers: Part I. Pharmacokinetics and clinical pharmacodynamics: Anesthesiology, 2020; 132(4); 636-51
15. Oka S, Satomi H, Sekino R, Sedation outcomes for remimazolam, a new benzodiazepine: J Oral Sci, 2021; 63(3); 209-11
16. Morimoto Y, Efficacy and safety profile of remimazolam for sedation in adults undergoing short surgical procedures: Ther Clin Risk Manag, 2022; 18; 95-100
17. Zhou Y, Hu P, Jiang J, Metabolite characterization of a novel sedative drug, remimazolam in human plasma and urine using ultra high-performance liquid chromatography coupled with synapt high-definition mass spectrometry: J Pharm Biomed Anal, 2017; 137; 78-83
18. Mao Y, Guo J, Yuan J, Quality of recovery after general anesthesia with remimazolam in patients’ undergoing urologic surgery: A randomized controlled trial comparing remimazolam with propofol: Drug Des Devel Ther, 2022; 16; 1199-209
19. Zhang J, Wang X, Zhang Q, Application effects of remimazolam and propofol on elderly patients undergoing hip replacement: BMC Anesthesiol, 2022; 22(1); 118
20. Guan HL, Liu H, Hu XY, Urinary albumin creatinine ratio associated with postoperative delirium in elderly patients undergoing elective non-cardiac surgery: A prospective observational study: CNS Neurosci Ther, 2022; 28(4); 521-30
21. Li YW, Li HJ, Li HJPeking University Clinical Research Program Study Group, Delirium in older patients after combined epidural-general anesthesia or general anesthesia for major surgery: A randomized trial: Anesthesiology, 2021; 135(2); 218-32
22. Liu T, Tuo J, Wei Q, Effects of abdominal wall blocks on postoperative delirium in elderly patients undergoing laparoscopic surgery: A randomized controlled study: Med Sci Monit, 2022; 28; e934281
23. Bugiani O, Why is delirium more frequent in the elderly?: Neurol Sci, 2021; 42(8); 3491-503
24. Liu H, Zhao Q, Liu X, Incidence and interaction factors of delirium as an independent risk of mortality in elderly patients in the intensive units: A retrospective analysis from MIMIC-IV database: Aging Clin Exp Res, 2022; 34(11); 2865-72
25. Zhou Y, Li Y, Wang K, Bispectral index monitoring during anesthesia promotes early postoperative recovery of cognitive function and reduces acute delirium in elderly patients with colon carcinoma: A prospective controlled study using the attention network test: Med Sci Monit, 2018; 24; 7785-93
26. Maheshwari K, Ahuja S, Khanna AK, Association between perioperative hypotension and delirium in postoperative critically ill patients: A retrospective cohort analysis: Anesth Analg, 2020; 130(3); 636-43
27. Chen F, Liu L, Wang Y, Delirium prevalence in geriatric emergency department patients: A systematic review and meta-analysis: Am J Emerg Med, 2022; 59; 121-28
28. Duprey MS, Dijkstra-Kersten SMA, Zaal IJ, Opioid use increases the risk of delirium in critically ill adults independently of pain: Am J Respir Crit Care Med, 2021; 204(5); 566-72
29. Vasunilashorn SM, Ngo LH, Jones RN, The association between c-reactive protein and postoperative delirium differs by Catechol-O-Methyltransferase genotype: Am J Geriatr Psychiatry, 2019; 27(1); 1-8
30. Ortiz D, Lindroth HL, Braly T, Delirium severity does not differ between medical and surgical intensive care units after adjusting for medication use: Sci Rep, 2022; 12(1); 14447
31. Marcantonio ER, Juarez G, Goldman L, The relationship of postoperative delirium with psychoactive medications: JAMA, 1994; 272(19); 1518-22
32. Wang E, Belley-Côté EP, Young J, Effect of perioperative benzodiazepine use on intraoperative awareness and postoperative delirium: A systematic review and meta-analysis of randomised controlled trials and observational studies: Br J Anaesth, 2023; 131(2); 302-13
33. Ouanes S, Popp J, High cortisol and the risk of dementia and Alzheimer’s disease: A review of the literature: Front Aging Neurosci, 2019; 11; 43
34. Zhang J, Cairen Z, Shi L, Remimazolam versus propofol for procedural sedation and anesthesia: A systemic review and meta-analysis: Minerva Anestesiol, 2022; 88(12); 1035-42
35. Zhang X, Li S, Liu J, Efficacy and safety of remimazolam besylate versus propofol during hysteroscopy: Single-centre randomized controlled trial: BMC Anesthesiol, 2021; 21(1); 156 [Erratum in: BMC Anesthesiol. 2021;21(1):173]
Tables
 Table 1. Demographic characteristics of patients in both groups.
Table 1. Demographic characteristics of patients in both groups. Table 2. Comparison of intraoperative and postoperative indexes between the 2 groups.
Table 2. Comparison of intraoperative and postoperative indexes between the 2 groups.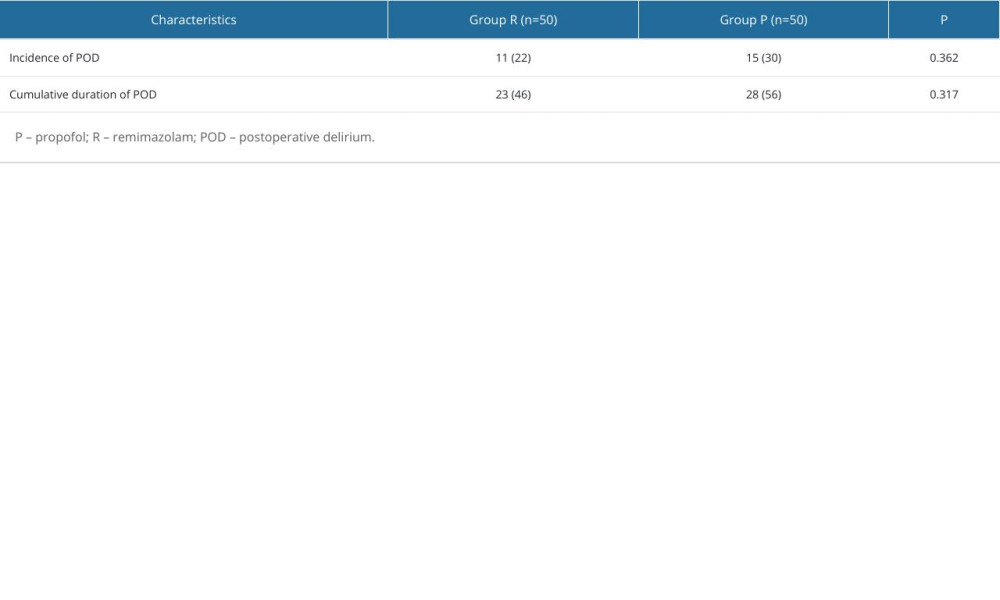 Table 3. Comparison of incidence and cumulative duration of postoperative delirium between the 2 groups [n (%)].
Table 3. Comparison of incidence and cumulative duration of postoperative delirium between the 2 groups [n (%)].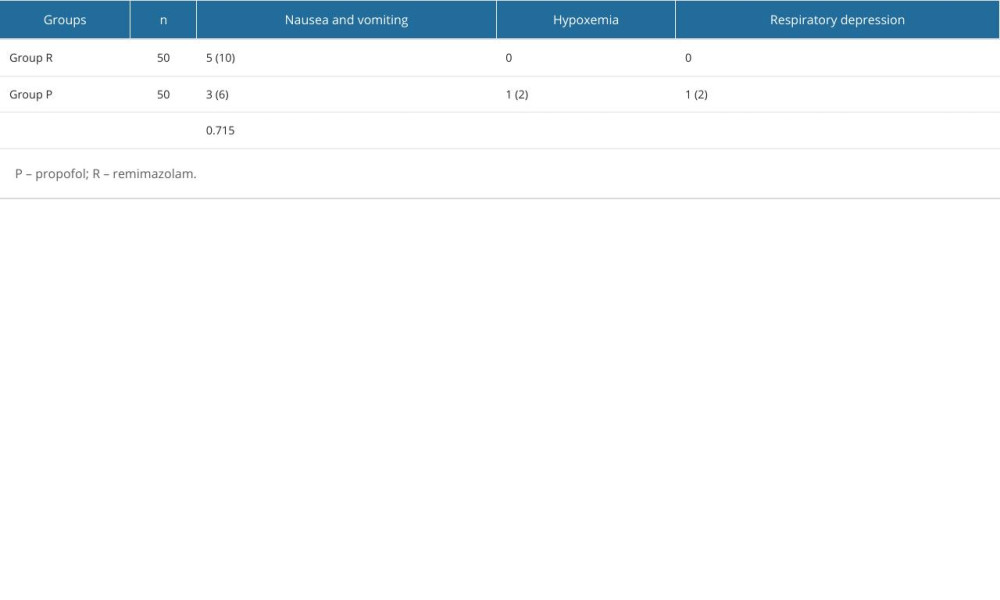 Table 4. The occurrence of adverse reactions in the 2 groups [n (%)].
Table 4. The occurrence of adverse reactions in the 2 groups [n (%)]. Table 1. Demographic characteristics of patients in both groups.
Table 1. Demographic characteristics of patients in both groups. Table 2. Comparison of intraoperative and postoperative indexes between the 2 groups.
Table 2. Comparison of intraoperative and postoperative indexes between the 2 groups. Table 3. Comparison of incidence and cumulative duration of postoperative delirium between the 2 groups [n (%)].
Table 3. Comparison of incidence and cumulative duration of postoperative delirium between the 2 groups [n (%)]. Table 4. The occurrence of adverse reactions in the 2 groups [n (%)].
Table 4. The occurrence of adverse reactions in the 2 groups [n (%)]. In Press
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
11 Mar 2024 : Clinical Research
Comparison of Effects of Sugammadex and Neostigmine on Postoperative Neuromuscular Blockade Recovery in Pat...Med Sci Monit In Press; DOI: 10.12659/MSM.942773
12 Mar 2024 : Clinical Research
Comparing Neuromuscular Blockade Measurement Between Upper Arm (TOF Cuff®) and Eyelid (TOF Scan®) Using Miv...Med Sci Monit In Press; DOI: 10.12659/MSM.943630
11 Mar 2024 : Clinical Research
Enhancement of Frozen-Thawed Human Sperm Quality with Zinc as a Cryoprotective AdditiveMed Sci Monit In Press; DOI: 10.12659/MSM.942946
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952