22 April 2024: Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Department
Michał MachowskiDOI: 10.12659/MSM.942612
Med Sci Monit 2024; 30:e942612
Abstract
BACKGROUND: COVID-19 increases the risk of acute cardiovascular diseases (CVDs), including acute coronary syndrome (ACS), acute pulmonary embolism (APE), and acute myocarditis (AMyo). The actual impact of CVDs on mortality of patients with COVID-19 remains unknown. This study aimed to determine whether CVDs influence the course of COVID-19 pneumonia and if they can be easily detected by using common tests and examinations.
MATERIAL AND METHODS: Data of 249 consecutive patients with COVID-19 hospitalized in a dedicated cardiology department were analyzed. On admission, clinical status, biomarkers, computed tomography, and bedside echocardiography were performed.
RESULTS: D-dimer level predicted APE (AUC=0.850 95% CI [0.765; 0.935], P<0.001) with sensitivity of 69.4% and specificity of 96.2% for a level of 4968.0 ng/mL, and NT-proBNP predicted AMyo (AUC=0.692 95% CI [0.502; 0.883], P=0.004) and showed sensitivity of 54.5%, with specificity of 86.5% for the cut-off point of 8970 pg/mL. Troponin T levels were not useful for diagnostic differentiation between CVDs. An extent of lung involvement predicted mortality (OR=1.03 95% CI [1.01;1.04] for 1% increase, P<0.001). After adjusting for lung involvement, ACS increased mortality, compared with COVID-19 pneumonia only (OR=5.27 95% CI [1.76; 16.38] P=0.003), while APE and AMyo did not affect risk for death.
CONCLUSIONS: D-dimer and NT-proBNP, but not troponin T, are useful in differentiating CVDs in patients with COVID-19. ACS with COVID-19 increased in-hospital mortality independently from extent of lung involvement, while coexisting APE or AMyo did not.
Keywords: Pulmonary Embolism, acute coronary syndrome, myocarditis, COVID-19
Introduction
Coronavirus infection results in SARS-CoV-2 binding to the angiotensin-converting enzyme 2 receptor to mediate entry into cells [1]. In addition to the lungs, the angiotensin-converting enzyme 2 receptor is highly expressed in the heart and blood vessels [2]. Severe cases of viral pneumonia, such as with SARS-CoV-2 infection in COVID-19, manifest by the progression of systemic inflammation and overactivation of immune cells with “cytokine storm”, which leads to cardiac damage and destabilization of atherosclerotic plaques with its consequences and predisposes to a hypercoagulable state [3–6]. A significant increase of the risk of acute cardiovascular diseases (CVDs), including acute coronary syndrome (ACS), acute pulmonary embolism (APE), and stroke in patients with COVID-19 were reported [3,7]. Acute myocarditis (AMyo) can also be caused by COIVD-19 [8]. Moreover, it was reported that COVID-19 increases in-hospital [9,10] and post-discharge [8] mortality of the aforementioned CVDs. Patients with heart failure admitted to hospital with concomitant SARS-CoV-2 infection had a very poor prognosis [11]. Moreover, recent analysis has shown that SARS-CoV-2 infection is associated with worse in-hospital mortality in patients with ACS [12] or in patients with APE [13]. Since SARS-CoV-2 infection affects mortality in CVDs it is crucial to optimize the diagnosis and management of this group of patients and to increase the awareness among medical professionals. Although COVID-19 increases the mortality rate of a wide spectrum of CVDs, studies directly comparing the clinical course of patients with COVID-19 with concomitant acute CVDs are very limited. Moreover, comparative analysis of clinical and biochemical characteristics of patients with various CVDs and COVID-19 could help to optimize the diagnostic workup and to assess predictors of short-term outcomes. We retrospectively analyzed the data of patients with or without acute CVDs, including ACS, APE, and AMyo, who were admitted and treated for COVID-19 pneumonia at a tertiary referral cardiac unit. The main aim of this study was to evaluate and identify specific diagnostic markers for different CVDs and to determine the potential impact of acute CVDs in patients with COVID-19 on hospital mortality.
Material and Methods
INITIAL ASSESSMENT OF PATIENTS WITH COVID-19:
On admission, all patients’ clinical status was assessed with the Modified Early Warning Score (MEWS) [14] and pulsoximetry. Clinical evaluations and the MEWS with pulsoximetry were performed at least twice daily. For any single physiological parameter scored, a higher level of care for the patient was considered.
In addition, routine assay laboratory tests included high-sensitive troponin T (hsTnT; Elecsys, Roche Diagnostics; reference range <14 ng/mL), D-dimer (Elecsys; reference range <500 ng/mL), N-terminal pro-brain natriuretic peptide (NT-proBNP; Elecsys; reference range <125 pg/mL), and procalcitonin (Elecsys; low risk of bacterial infection <0.25 ng/mL).
Standard electrocardiogram was recorded with an electrocardiographic system (Philips Medical System, Page Writer Trim III, Andover, MA, USA). Tracings were acquired at 25 mm/s, 50 mm/s paper speed, and 10 mm/mV gain. On admission, all patients underwent chest computed tomography (CT) with assessment of percentage of COVID-19 lung involvement. If the D-dimer plasma level was above the age-adjusted norm, additional pulmonary angiogram (CTPA) was performed for the diagnosis of suspected APE. Subsequently, all patients underwent bedside echocardiography.
DIAGNOSIS OF CVDS:
APE was diagnosed when at least a segmental thrombus was visualized at CTPA. In patients with ambiguous CTPA results, compression ultrasound was performed for deep vein thrombosis.
ACS was diagnosed following the current definition of myocardial infraction published by the European Society of Cardiology [14,15]. All patients with suspected ACS were referred for urgent coronary angiography and for percutaneous coronary intervention when indicated. Takotsubo cardiomyopathy was diagnosed after exclusion of myocardial infarction, in the setting of changes at electrocardiography suggestive for ischemia and at echocardiography as described in a consensus paper: when echocardiography showed apical ballooning, or hypo-, a-, or dyskinesia of mid-apical myocardial segments were typical or associated with hypokinetic mid-segments [16].
AMyo was suspected when at least a moderately elevated plasma hsTnT level (>25 ng/mL) was detected, especially in patients with acute heart failure or cardiac arrhythmias, or when new wall motion abnormalities were echocardiographically diagnosed, with left ventricle ejection fraction (LVEF) below 40% [3]. Patients with suspected AMyo underwent coronary angiography to exclude ACS. To verify the diagnosis, all of these patients were scheduled for post-discharge cardiovascular magnetic resonance imaging.
COMPUTED TOMOGRAPHY:
CT was performed in the Emergency Department using a 128-slice scanner (Ingenuity Core, Philips, Amsterdam, The Netherlands). The patients were scanned during a full-inspiration breath-hold in a supine position with the arms above the head. The scan area extended from the lung apices to the adrenal glands. For CTPA, approximately 80 mL (1.2 mL/kg) of contrast medium (Omnipaque 350) was administered intravenously with an automatic power injector, at the rate of 5.5 mL/s. The scanning was initiated with a bolus tracking technique when the enhancement in the pulmonary trunk reached 130 HU. The scanning parameters were as follows: reconstructed slice thickness of 0.5 mm, pitch of 1.5, rotation time of 0.4 s, 100 kVp, and automatic mAs modulation.
ECHOCARDIOGRAPHY:
On admission, bedside echocardiography was performed with the Philips VIVID Q CX5 system. The protocol focused on the assessment of right ventricular dysfunction, global and regional left ventricular systolic function, and major valvular lesions. Right ventricular dysfunction was diagnosed when the right ventricular end-diastolic dimension to the left ventricular end-diastolic dimension assessed in the 4-chamber apical or subcostal view exceeded 1.0, and the tricuspid valve peak systolic gradient exceeded 30 mm Hg. To calculate LVEF, the Simpson method was used.
MANAGEMENT:
The aim of oxygen therapy was to maintain peripheral blood oxygen saturation (SpO2) above 95% in the general patient population, and above 88% in patients with chronic hypercapnia. Conventional forms of oxygen supplementation included nasal cannulas and face masks. High-flow nasal cannulas or noninvasive ventilation were used when these methods failed to reached desired SpO2, combined with excessive respiratory effort, defined as >20 breathes/min.
If the abovementioned methods failed to obtain the desired parameters, with further deterioration of respiratory failure, mechanical ventilation or qualification for extracorporeal mechanical oxygenation were introduced.
Remdesivir was given intravenously once daily, the first day at 200 mg, followed by 100 mg daily for 4 days; dexamethasone 6 mg once daily was given orally or intravenously for up to 10 days or until discharge. Procalcitonin levels were assayed at admission and when the patient did not improve. In patients with procalcitonin levels above 0.25 ng/mL, antibiotic therapy was started immediately. All patients who did not require anticoagulation for other reasons received thromboprophylaxis with low molecular weight heparin, unless contraindicated.
Patients diagnosed with APE were treated according to the European Society of Cardiology recommendations [17–19]. Patients with ACS received typical treatment, according to the European Society of Cardiology guidelines [9,15,20].
STATISTICAL ANALYSIS:
We performed descriptive analysis followed by group comparisons. Subsequently, predictors for death and mechanical ventilation were identified with logistic regression analysis. There were prepared non-adjusted and adjusted models (COVID-19 lung involvement at CT as a covariate). Receiver operating characteristic (ROC) curve analysis was done to find the optimal cut-off points for selected parameters as predictors for the diagnosis of CVDs. Cut-off point calculation was based on the Youden index.
Data are presented as number (%) for discrete data and as mean (SD) or median (Q1;Q3) for continuous data, as appropriate. Normality of distribution was verified with the Shapiro-Wilk test, skewness, and kurtosis values. Comparison of groups was made with the chi-square test, Fisher exact test, ANOVA, or Kruskal-Wallis test, as appropriate. In case of significant differences in primary analyses, post-hoc tests were calculated: Tukey test for ANOVA and Dunn test for Kruskal-Wallis test (with Bonferroni correction for multiple comparisons). Predictors for death and mechanical ventilation were identified with logistic regression analysis, with data presented by odds ratios (OR) with 95% confidence intervals (CI) and
Analysis was conducted in R software, version 4.0.5 (R Core Team [2021]. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria), assuming a significance level α=0.05.
Results
ANALYSIS OF PATIENT WITH COVID-19 ACCORDING TO COEXISTING CVDS:
All groups were similar in age and sex and presented similar MEWS scores on admission (Table 2). However, the proportions of previously diagnosed coronary artery disease (CAD) and congestive heart failure (CHF) were significantly different (P<0.001 and P=0.004, respectively). History of CAD was more frequent in patients with ACS (40%) and AMyo (52.6%) than in the APE and COVID-19-only groups (14.3% and 18.9%, respectively). Previously diagnosed CHF was most frequently present in AMyo (47.4%), compared with in patients with APE (7.1%), ACS (25.0%), and COVID-19 only (19.6%). Differences in the extent of lung involvement caused by COVID-19 pneumonia between analyzed groups were observed. Patients with APE and patients with COVID-19 pneumonia presented significantly higher lung involvement than did patients with ACS.
LABORATORY AND ECHOCARDIOGRAPHIC DATA ACCORDING TO COEXISTING CVDS:
There were significant differences between the groups in plasma levels of hsTnT, NT-proBNP, and D-dimer (P=0.002 for NT-proBNP, and P<0.001 in remaining biomarkers, Table 3). Post-hoc analysis revealed that hsTnT and NT-proBNP levels were higher in patients with ACS (hsTnT, 153.5 ng/mL; NT-proBNP, 4200 pg/mL) and AMyo (hsTnT, 75.5 ng/mL; NT-proBNP, 9083 pg/mL) than in patients with APE (hsTnT, 26.0 ng/mL; NT-proBNP, 962 pg/mL) or COVID-19 pneumonia (hsTnT, 22.5 ng/mL; NT-proBNP, 996 pg/mL), whereas D-dimer levels were higher in patients with APE (12 675 ng/mL) than in patients with AMyo (1317 ng/mL) and COVID-19 (959 ng/mL). However, we observed no differences in D-dimer levels between patients with APE and ACS (1494 ng/mL).
Echocardiography showed that LVEF was lower in patients with AMyo than in those with ACS. Of note, right ventricular dysfunction was observed in 47.6% of patients with APE and in no patients with ACS or AMyo. However, right ventricular dysfunction was also detected in 3 patients with COVID-19 pneumonia in whom APE was excluded.
Medians and means for biomarkers in each group are presented in Table 2.
ROC ANALYSIS:
To identify the optimal cut-off points for selected parameters as predictors for the diagnosis of APE, ACS, and AMyo, we conducted ROC curve analysis (Table 4), which showed that D-dimer was a significant predictor for APE for a cut-off of 4968.0 ng/mL, with sensitivity of 69.4%, specificity of 96.2%, PPV of 80.6%, and NPV of 93.3% (95% CI 0.765; 0.935). NT-proBNP was a significant predictor for the diagnosis of AMyo for the cut-off point of 8970 pg/mL, with sensitivity of 54.5%, specificity of 86.5%, PPV of 29.8%, and NPV of 88% (95% CI 0.502; 0.883). Values of hsTnT were not useful for diagnostic differentiation between the studied groups.
LVEF was a significant predictor for AMyo diagnosis for a cut-off point of 33.5%, with sensitivity of 86.7%, specificity of 91.2% (95% CI 0.899; 0.996), NPV of 61.9%, and PPV of 91.6%.
Right ventricular dysfunction at echocardiography was a diagnostic test for APE (Table 3).
CLINICAL COURSE AND OUTCOMES:
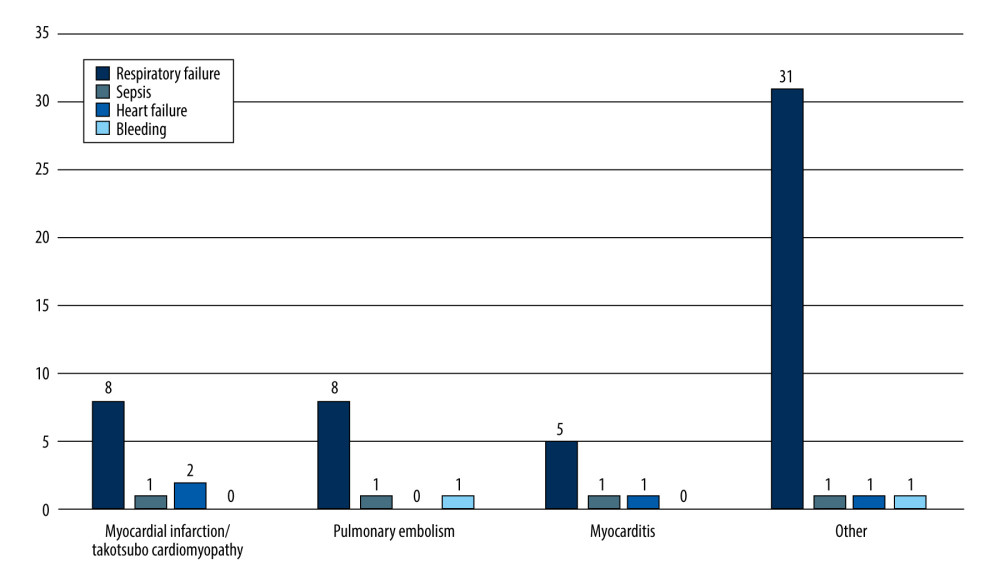
In total, 40 (16.1%) patients of the whole group required mechanical ventilation due to progression of respiratory failure, and 8 of them survived. In total, there were 60 (24.1%) in-hospital deaths (Table 5). This group included 10 patients with ACS (in-hospital mortality 25.0%), 9 patients with APE (21.4%), 7 patients with AMyo (36.8%), and the remaining 34 patients with COVID-19 pneumonia without aforementioned CVDs. Figure 1 shows the breakdown of causes of death in the study groups. The most frequent cause of death was irreversible respiratory failure (49 patients). However, 4 deaths were caused by sepsis, 4 patients died due to severe heart failure and cardiogenic shock, and 3 patients experienced fatal bleeding. Of note, we found no differences in either the use of mechanical ventilation or in-hospital mortality between the studied groups. Interestingly, hospitalization time was significantly shorter in the ACS (3.5 days) group than in the APE or COVID-19 subgroups (9.0 and 10.0 days, respectively; P<0.001).
EFFECT OF COEXISTING ACUTE CARDIOVASCULAR DISEASE ON IN-HOSPITAL MORTALITY AND MECHANICAL VENTILATION:
We analyzed the potential effect of acute CVDs (ACS, APE, AMyo) on survival. Patients with COVID-19 pneumonia only were regarded as the reference group (Table 6). A non-adjusted model showed no effect on all-cause in-hospital mortality of any of the analyzed acute CVDs. However, since there were significant differences in the extent of lung involvement of COVID-19 pneumonia by CT between groups (P=0.005), and lung involvement was a prognostic factor of in-hospital mortality in the whole group (OR=1.03, 95% CI [1.01; 1.04] for increase by 1%, P<0.001), an adjusted model was prepared including COVID-19 lung involvement as a covariate. Thus, when considering the extent of COVID-19 lung involvement, ACS was found to increase the risk of death, as compared with COVID-19 pneumonia only, with OR=5.27, 95% CI (1.76; 16.38) P=0,003, while APE and AMyo did not significantly affect odds for death, both in the non-adjusted and adjusted models. None of the diagnoses, both in non-adjusted and adjusted models, increased the risk of mechanical ventilation when compared with the group of patients with COVID-19 pneumonia only.
Discussion
STUDY LIMITATIONS:
This was an observational, retrospective, single-center study with the inherent limitations of this type of design and had a relatively limited number of patients, especially in the analyzed subpopulation with coexisting acute CVDs. Retrospective studies, when compared with prospective studies, provide an inferior level of evidence, as we had no control group.
Patients with elevated D-dimer levels on admission were routinely diagnosed for APE; however, ultrasound for deep vein thrombosis was performed only in cases of clinical suspicion or in cases of suspected APE when CTPA was inconclusive. AMyo was suspected when plasma levels of hsTnT higher than 25 ng/mL were detected, especially in patients with acute heart failure or cardiac arrhythmias, or when new wall motion abnormalities were echocardiographically diagnosed. However, elevated hsTnT levels were observed in 87.5% of the whole studied population, and hsTnT in the range of 15 to 25 ng/mL with preserved LVEF was present in 41 patients. It cannot be excluded that milder forms of myocarditis were not identified and thus it remains underdiagnosed. However, even milder forms of myocarditis may also have impacted the clinical course.
Another potential limitation of the study is that, at the time of the COVID-19 pandemic, we had no access to extracorporeal mechanical oxygenation. Although all patients received appropriate oxygen supplementation, better access to this technique might have improved the clinical outcome of some patients with severe respiratory failure.
CLINICAL IMPLICATIONS:
According to our data, it seems that patients with COVID-19 with concomitant CVDs optimally should be treated in dedicated cardiology departments. In such settings, the clinical outcomes of patients with COVID-19 with APE or myocarditis have a similar clinical course as that of patents with COVID-19 pneumonia only. However, COVID-19 patients with ACS form a high-risk group, independently from the extent of lung involvement and should be monitored and treated with special care.
Conclusions
D-dimer and NT-proBNP levels, while not hsTnT, are useful in differentiating CVDs in patients with COVID-19. We found that COVID-19 complicated by ACS increased in-hospital mortality independently from extent of lung involvement, while coexisting APE or AMyo did not.
Tables
Table 1. Initial clinical and laboratory characteristics of total study group.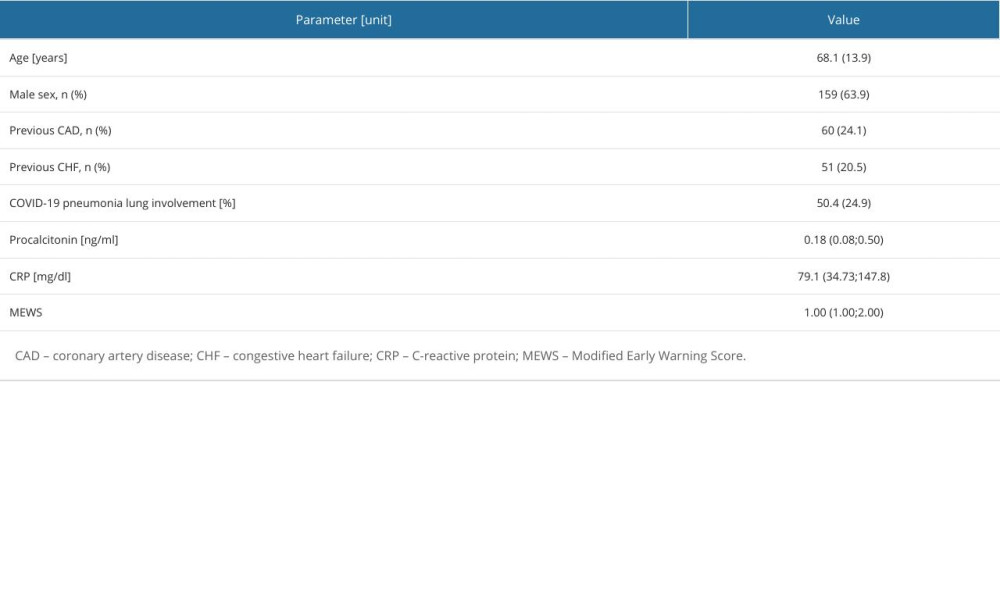 Table 2. Clinical characteristics according to the diagnosed acute cardiovascular disease.
Table 2. Clinical characteristics according to the diagnosed acute cardiovascular disease.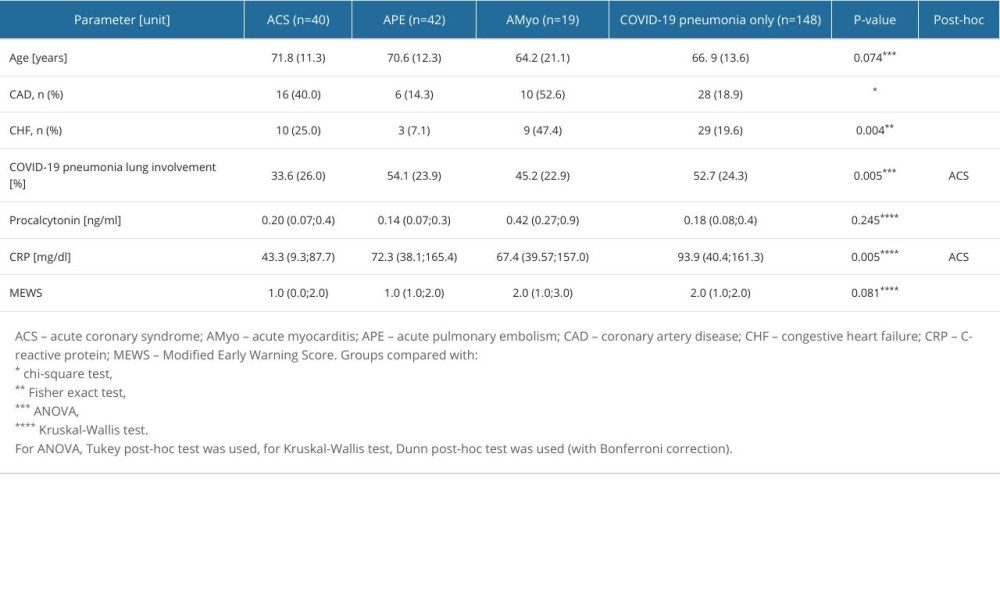 Table 3. Cardiac biomarkers and echocardiographic data according to the diagnosed acute cardiovascular diseases.
Table 3. Cardiac biomarkers and echocardiographic data according to the diagnosed acute cardiovascular diseases.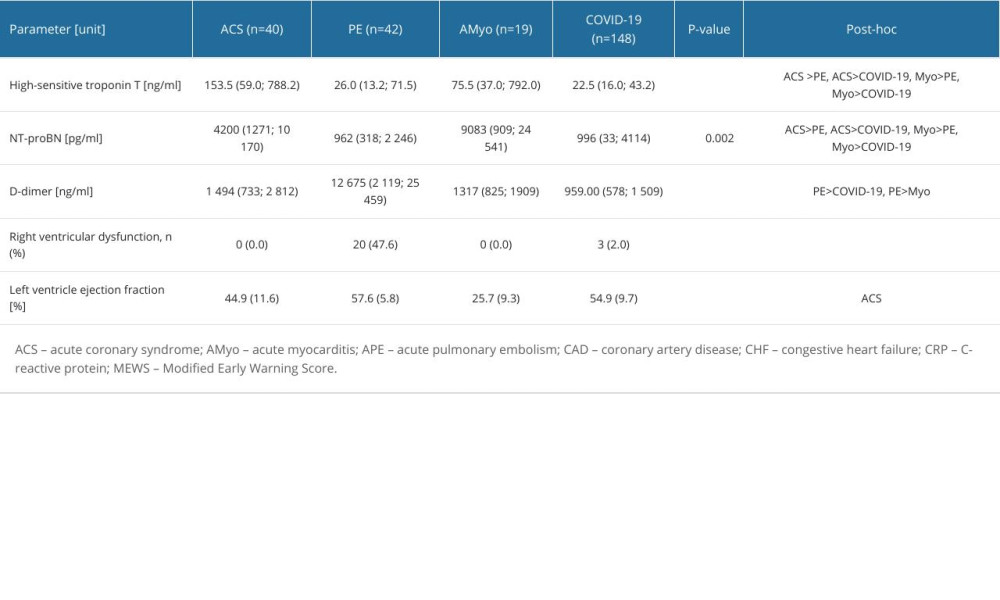 Table 4. Optimal cut-off points for the diagnosis of acute cardiovascular diseases.
Table 4. Optimal cut-off points for the diagnosis of acute cardiovascular diseases.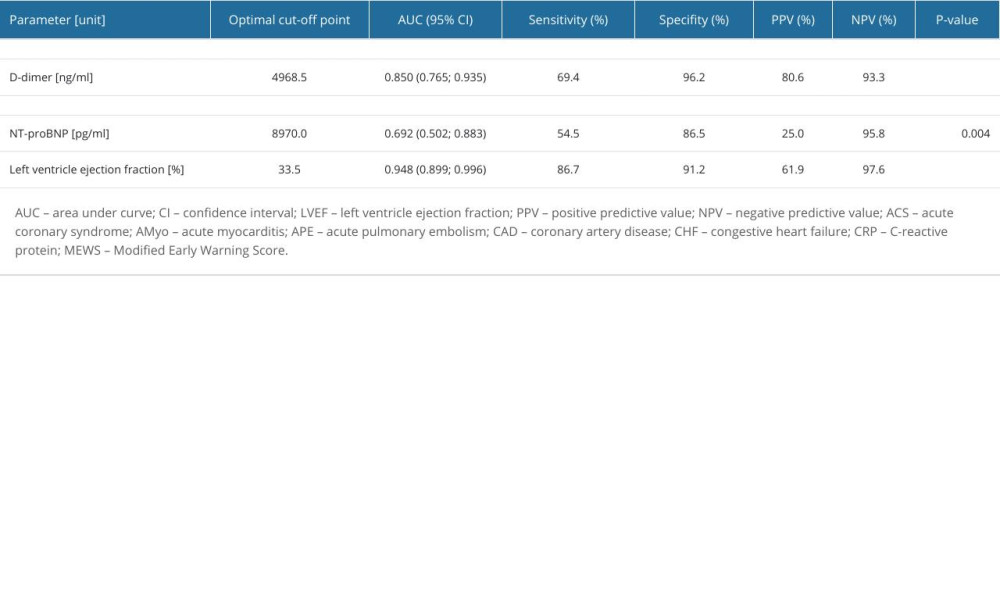 Table 5. Clinical course and outcome according to coexisting cardiovascular diseases.
Table 5. Clinical course and outcome according to coexisting cardiovascular diseases.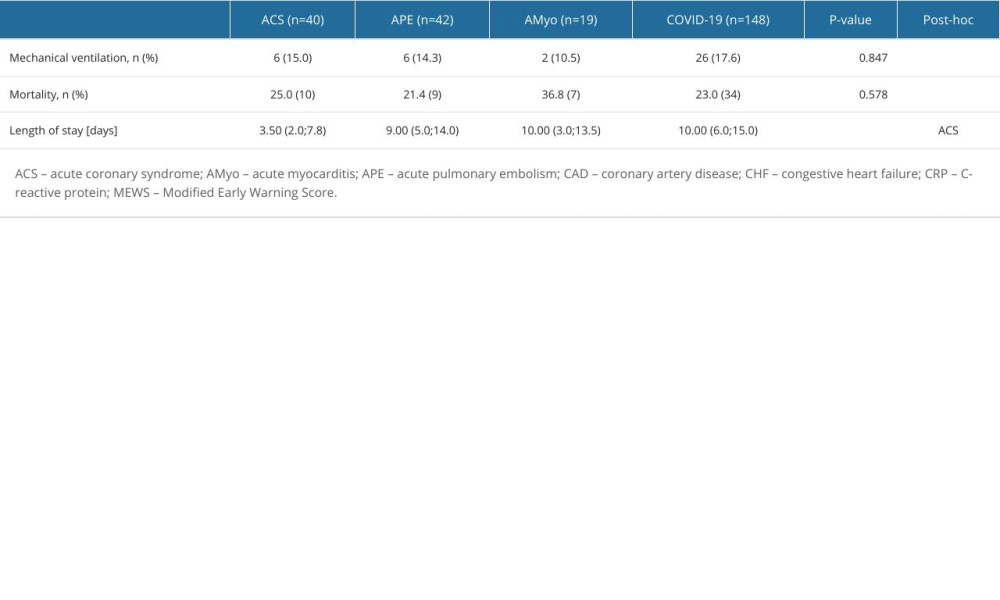 Table 6. Logistic regression for risk of death and mechanical ventilation in acute cardiovascular diseases, vs patients with COVID-19 pneumonia only.
Table 6. Logistic regression for risk of death and mechanical ventilation in acute cardiovascular diseases, vs patients with COVID-19 pneumonia only.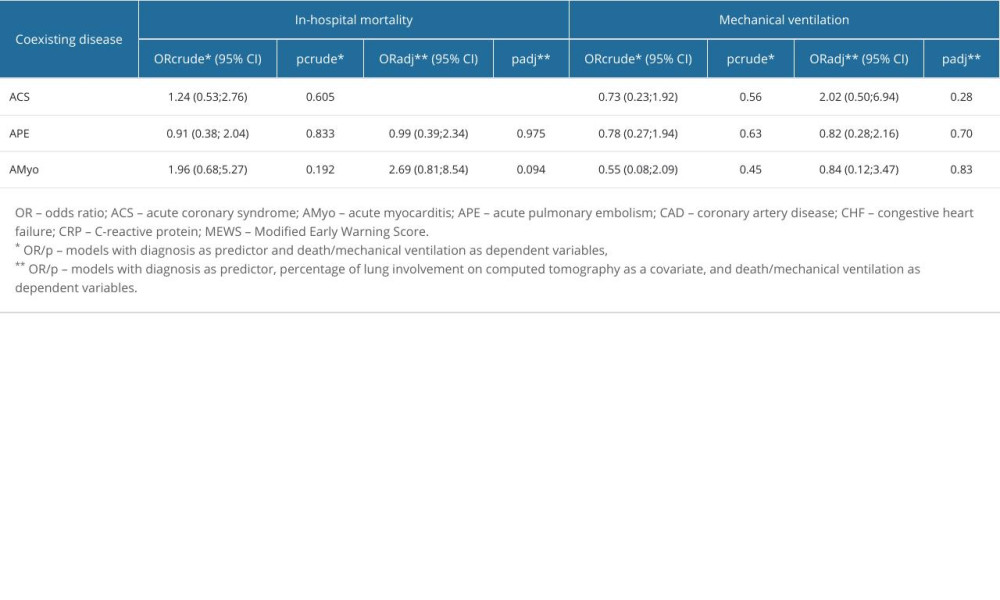
References
1. Hoffmann M, Kleine-Weber H, Schroeder S, SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor: Cell, 2020; 181(2); 271-80e8
2. Hamming I, Timens W, Bulthuis ML, Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis: J Pathol, 2004; 203(2); 631-37
3. Task Force for the management of COVID-19 of the European Society of Cardiology, European Society of Cardiology guidance for the diagnosis and management of cardiovascular disease during the COVID-19 pandemic: Part 1 – epidemiology, pathophysiology, and diagnosis: Eur Heart J, 2022; 43(11); 1033-58 Erratum in: Eur Heart J. 2021;34791157
4. Suh YJ, Hong H, Ohana M, Pulmonary embolism and deep vein thrombosis in COVID-19: A systematic review and meta-analysis: Radiology, 2021; 298(2); E70-E80
5. Modin D, Claggett B, Sindet-Pedersen C, Acute COVID-19 and the incidence of ischemic stroke and acute myocardial infarction: Circulation, 2020; 142(21); 2080-82
6. Connors JM, Levy JH, COVID-19 and its implications for thrombosis and anticoagulation: Blood, 2020; 135(23); 2033-40
7. Bojkova D, Wagner JUG, ShumLiakivska M, SARS-CoV-2 infects and induces cytotoxic effects in human cardiomyocytes: Cardiovasc Res, 2020; 116(14); 2207-15
8. Luetkens JA, Isaak A, Zimmer S, Diffuse myocardial inflammation in COVID-19 associated myocarditis detected by multiparametric cardiac magnetic resonance imaging: Circ Cardiovasc Imaging, 2020; 13(5); e010897
9. Task Force for the management of COVID-19 of the European Society of Cardiology, ESC guidance for the diagnosis and management of cardiovascular disease during the COVID-19 pandemic: Part 2 – care pathways, treatment, and follow-up: Eur Heart J, 2022; 43(11); 1059-103
10. Kite TA, Ludman PF, Gale CP, International prospective registry of acute coronary syndromes in patients with COVID-19: J Am Coll Cardiol, 2021; 77(20); 2466-76
11. Chatrath N, Kaza N, Pabari PA, The effect of concomitant COVID-19 infection on outcomes in patients hospitalized with heart failure: ESC Heart Fail, 2020; 7(6); 4443-47
12. Markson FE, Akuna E, Lim CY, The impact of COVID-19 on hospitalization outcomes of patients with acute myocardial infarction in the USA: Am Heart J Plus, 2023; 32; 100305
13. Hobohm L, Sagoschen I, Barco S, COVID-19 infection and its impact on case fatality in patients with pulmonary embolism: Eur Respir J, 2023; 61(1); 2200619
14. Subbe CP, Kruger M, Rutherford P, Validation of a modified Early Warning Score in medical admissions: QJM, 2001; 94(10); 521-26
15. Collet JP, Thiele H, Barbato E, 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Eur Heart J, 2021; 42(14); 1289-367
16. Ghadri JR, Wittstein IS, Prasad A, International expert consensus document on takotsubo syndrome (Part II): Diagnostic workup, outcome, and management: Eur Heart J, 2018; 39(22); 2047-62
17. Baigent C, Windecker S, Andreini DTask Force for the management of COVID-19 of the European Society of Cardiology, European Society of Cardiology guidance for the diagnosis and management of cardiovascular disease during the COVID-19 pandemic: Part 1 – epidemiology, pathophysiology, and diagnosis: Cardiovasc Res, 2022; 118(6); 1385-412
18. Konstantinides SV, Meyer G, Becattini C, 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): Eur Heart J, 2020; 41(4); 543-603
19. Kosior DA, Undas A, Kopec G, Guidance for anticoagulation management in venous thromboembolism during the coronavirus disease 2019 pandemic in Poland: An expert opinion of the Section on Pulmonary Circulation of the Polish Cardiac Society: Kardiol Pol, 2020; 78(6); 642-46
20. Ibanez B, James S, Agewall S, 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC): Eur Heart J, 2018; 39(2); 119-77
21. Machowski M, Polanska A, Galecka-Nowak M, Age-adjusted D-dimer levels may improve diagnostic assessment for pulmonary embolism in COVID-19 patients: J Clin Med, 2022; 11(12); 3298
22. Xu H, Martin A, Singh A, Pulmonary embolism in patients hospitalized with COVID-19 (from a New York Health System): Am J Cardiol, 2020; 133; 148-53
23. Gray WK, Navaratnam AV, Day J, COVID-19 hospital activity and in-hospital mortality during the first and second waves of the pandemic in England: An observational study: Thorax, 2022; 77(11); 1113-20
24. Sapienza LG, Nasra K, Calsavara VF, Risk of in-hospital death associated with COVID-19 lung consolidations on chest computed tomography – a novel translational approach using a radiation oncology contour software: Eur J Radiol Open, 2021; 8; 100322
25. Malecot N, Chrusciel J, Sanchez S, Chest CT characteristics are strongly predictive of mortality in patients with COVID-19 pneumonia: A multicentric cohort study: Acad Radiol, 2022; 29(6); 851-60
26. Li K, Chen D, Chen S, Predictors of fatality including radiographic findings in adults with COVID-19: Respir Res, 2020; 21(1); 146
27. Legutko J, Kleczynski P, Guzik B, Intracoronary and left ventricular thrombi in a 29-year-old COVID-19 convalescent with ST-segment elevation myocardial infarction: Kardiol Pol, 2023; 81(5); 535-36
28. Rosovsky RP, Grodzin C, Channick R, Diagnosis and treatment of pulmonary embolism during the coronavirus disease 2019 pandemic: A position paper from the National PERT Consortium: Chest, 2020; 158(6); 2590-601
29. Laouan Brem F, Asmae B, Amane Y, Diagnostic accuracy of D-dimers for predicting pulmonary embolism in COVID-19-patients: Clin Appl Thromb Hemost, 2021; 27; 10760296211057901
30. Bova C, Greco F, Misuraca G, Diagnostic utility of echocardiography in patients with suspected pulmonary embolism: Am J Emerg Med, 2003; 21(3); 180-83
31. Satoskar MA, Metkus T, Soleimanifard A, Improving risk prediction for pulmonary embolism in COVID-19 patients using echocardiography: Pulm Circ, 2022; 12(1); e12036
32. Mahmoud-Elsayed HM, Moody WE, Bradlow WM, Echocardiographic findings in patients with COVID-19 pneumonia: Can J Cardiol, 2020; 36(8); 1203-7
Tables
 Table 1. Initial clinical and laboratory characteristics of total study group.
Table 1. Initial clinical and laboratory characteristics of total study group. Table 2. Clinical characteristics according to the diagnosed acute cardiovascular disease.
Table 2. Clinical characteristics according to the diagnosed acute cardiovascular disease. Table 3. Cardiac biomarkers and echocardiographic data according to the diagnosed acute cardiovascular diseases.
Table 3. Cardiac biomarkers and echocardiographic data according to the diagnosed acute cardiovascular diseases. Table 4. Optimal cut-off points for the diagnosis of acute cardiovascular diseases.
Table 4. Optimal cut-off points for the diagnosis of acute cardiovascular diseases. Table 5. Clinical course and outcome according to coexisting cardiovascular diseases.
Table 5. Clinical course and outcome according to coexisting cardiovascular diseases. Table 6. Logistic regression for risk of death and mechanical ventilation in acute cardiovascular diseases, vs patients with COVID-19 pneumonia only.
Table 6. Logistic regression for risk of death and mechanical ventilation in acute cardiovascular diseases, vs patients with COVID-19 pneumonia only. Table 1. Initial clinical and laboratory characteristics of total study group.
Table 1. Initial clinical and laboratory characteristics of total study group. Table 2. Clinical characteristics according to the diagnosed acute cardiovascular disease.
Table 2. Clinical characteristics according to the diagnosed acute cardiovascular disease. Table 3. Cardiac biomarkers and echocardiographic data according to the diagnosed acute cardiovascular diseases.
Table 3. Cardiac biomarkers and echocardiographic data according to the diagnosed acute cardiovascular diseases. Table 4. Optimal cut-off points for the diagnosis of acute cardiovascular diseases.
Table 4. Optimal cut-off points for the diagnosis of acute cardiovascular diseases. Table 5. Clinical course and outcome according to coexisting cardiovascular diseases.
Table 5. Clinical course and outcome according to coexisting cardiovascular diseases. Table 6. Logistic regression for risk of death and mechanical ventilation in acute cardiovascular diseases, vs patients with COVID-19 pneumonia only.
Table 6. Logistic regression for risk of death and mechanical ventilation in acute cardiovascular diseases, vs patients with COVID-19 pneumonia only. In Press
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
11 Mar 2024 : Clinical Research
Comparison of Effects of Sugammadex and Neostigmine on Postoperative Neuromuscular Blockade Recovery in Pat...Med Sci Monit In Press; DOI: 10.12659/MSM.942773
12 Mar 2024 : Clinical Research
Comparing Neuromuscular Blockade Measurement Between Upper Arm (TOF Cuff®) and Eyelid (TOF Scan®) Using Miv...Med Sci Monit In Press; DOI: 10.12659/MSM.943630
11 Mar 2024 : Clinical Research
Enhancement of Frozen-Thawed Human Sperm Quality with Zinc as a Cryoprotective AdditiveMed Sci Monit In Press; DOI: 10.12659/MSM.942946
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952