04 May 2024: Database Analysis
Risk Factors of Age-Related Macular Degeneration in a Population-Based Study: Results from SHIP-TREND-1 (Study of Health in Pomerania-TREND-1)
Lisa LüdtkeDOI: 10.12659/MSM.943140
Med Sci Monit 2024; 30:e943140
Abstract
BACKGROUND: Age-related macular degeneration (AMD) is the most common cause of visual impairment in the elderly population in industrialized countries. The Study of Health in Pomerania (SHIP) with its cohort SHIP-TREND was designed to investigate risk factors and clinical disorders in the general population of northeast Germany. This work focused on the first follow-up of SHIP-TREND and determined associated modifiable risk factors of AMD. Modifying risk factors is important to slow the progression of early AMD as there is currently no treatment for the late stage of geographic atrophy. Understanding AMD-associated risk factors also plays an important role in the development of therapeutic concepts.
MATERIAL AND METHODS: Between 2016 and 2019, data were collected from a total of 2507 initially randomly selected subjects from the general population aged 28 to 89 years. Non-mydriatic fundus photography of the right eye was performed in 2489 subjects. Grading of AMD was performed using the Rotterdam classification system.
RESULTS: We included 1418 gradable fundus photographs in the analysis. The risk of AMD changes increased with age and was positively correlated with HDL cholesterol, fT3, and low educational level. In men, BMI and cigarette smoking were also positively associated with AMD changes.
CONCLUSIONS: This study emphasizes the consideration of various metabolic pathways for the development of therapeutic concepts.
Keywords: Epidemiologic Studies, Epidemiology, Macular Degeneration, Photograph
Introduction
Age-related macular degeneration (AMD) is one of the major causes of visual impairment or blindness in advanced adulthood in industrialized countries. AMD is characterized by the appearance of pigment epithelial changes and drusen (ie, yellow deposits in the pigment epithelium) [1] (Figure 1A). These changes progress to the late stage of geographic atrophy (Figure 1B) (ie, complete loss of pigment epithelium and photoreceptors in the macular area or the formation of neovascularization, which leads to fluid accumulation in and under the retina). This accumulation of fluid is also referred to as wet or exudative AMD, while geographic atrophy describes late-stage dry AMD [2,3].
People affected by AMD report increasing difficulties in reading and the need for good lighting. As the condition progresses, central scotomas and impaired vision may occur [2]. Advanced and late stages of AMD in particular are associated with significantly reduced visual acuity. The frequency of depression was found to double in these patients, which is exacerbated by the loss of independence and leisure activities [3,4].
It is known that AMD is based on a multifactorial process [3,5]. In addition to genetic factors, such as a polymorphism in the gene for complement factor H (CFH) gene [6], age and processes that promote oxidative stress [7,8] also play a role. The most important risk factor in this context is smoking [5]. Other risk factors are obesity, diet, and cardiovascular risk factors [9] such as coronary artery stenosis [10] or light exposure [7,8]. The diagnosis of AMD is based on the clinical examination or assessment of fundus photographs. In addition, ocular coherence tomography (OCT) and fluorescein angiography are available to confirm the diagnosis [2]. While there is currently no treatment concept for dry AMD, exudative AMD can be treated by intravitreal administration of anti-VEGF inhibitors [2]. The management of dry AMD focusses on adjustment of risk factors and use of dietary supplements [11,12].
In Germany, the prevalence of early AMD increased from 5.7 million to around 7 million between 2002 and 2017. The late stages showed an increase from 360 000 to 490 000 affected people in the same period [13]. It is estimated that 200 million people worldwide had AMD in 2020 [14]. Due to progressive demographic changes, this number is expected to increase in the coming decades. Wong et a made a prognostic projection of the possible development until 2040. In this projection, the number of patients affected by AMD will increase from 196 million in 2020 to 288 million in 2040 [14]. Due to demographic change, the number of patients affected by AMD is expected to increase in the coming years. The prognosis by Wong et al illustrates this fact. Patients experience a gradual loss of vision, which leads to impairments in daily life and in social contacts. The rate of depression in these patients is reported to be twice as high as in unaffected people of the same age [4]. Patients with advanced macular changes are increasingly dependent on help in everyday life and the currently available therapies are expensive, also due to the need for long-term treatment in many cases. It is therefore important to collect further information on risk factors associated with AMD to optimize the adjustment of risk factors, especially in the context of dry AMD, which is not yet treatable, and to contribute to the understanding of AMD itself and the development of therapy concepts.
AMD and associated risk factors have already been investigated and described in various studies worldwide [15–22]. The prevalence of AMD and its associated risk factors in the SHIP-TREND baseline study (SHIP-TREND-0) has already been described [23]. The prevalence of AMD for early and late forms is comparable to other studies from Europe, USA, Australia, and Asia with analysis of fundus images [15–17,24]. In the analysis of the SHIP-TREND-0, early changes in the form of retinal pigment epithelial changes or drusen occurred more frequently in comparison with other national and international studies [23]. In addition to age, variables related to obesity were found to be associated with AMD. These include an increased BMI, waist circumference, and hip circumference. Smoking, cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, HbA1c, diet, and cardiovascular disease were not associated [23].
The aim of this study was to verify the results of the risk factors from SHIP-TREND-0 and to identify additional risk factors in SHIP-TREND-1 to provide further information for understanding the disease and developing therapeutic concepts.
Material and Methods
STUDY DESIGN AND POPULATION:
SHIP is a population-based health project in northeast Germany. SHIP-TREND is the second cohort of this study in total and was designed to collect information on risk factors, subclinical findings, and diseases and to investigate complex relationships among these data. In SHIP-TREND-0, an examination of the fundus of the right eye of each participant was carried out using non-mydriatic fundus photography. These baseline data were collected between 2008 and 2012 and included 4420 subjects 20–79 years of age. The subjects were recruited using an age- and sex-stratified random sample according to age and sex of the residents’ registration offices from the cities of Stralsund, Greifswald, and Anklam and the surrounding municipalities. All procedures performed were in accordance with the ethics standards of the institutional research committee of University Medicine Greifswald (former Ernst-Moritz-Arndt-University) and with the 1964 Helsinki Declaration and its later amendments. All participants were informed about the purpose and assessments of the study, and gave their written informed consent.
For the first follow-up examination (SHIP-TREND-1), all subjects who were still alive were invited to participate and data were collected between 2016 and 2019. In total, follow-up data were collected from 2507 subjects aged 28–89 years. Eighteen subjects had to be excluded due to missing ophthalmological examination and another 31 subjects were excluded due to missing covariates such as missing age, sex, or other variables. Thus, 2458 fundus images were available for AMD grading; 1040 subjects had to be excluded due to reduced image quality, leaving 1418 fundus images available for this analysis (Figure 2). The mean age was 57.1 years. The proportion of female subjects was 51.6%.
FUNDUS PHOTOGRAPHY:
A 45° standardized fundus image of the right eye, centered on the optic disc, was performed by a trained and certified study nurse using a TCR-NW 200 non-mydriatic camera (Topcon Corporation, Tokyo, Japan). The examination was performed in a darkened room to allow natural pupil dilation. Dark adaptation took place for a few minutes. Images were recorded using Visualis 2.62 imaging software (Imedos, Jena, Germany). The ophthalmological examinations and all the other assessments are described in detail elsewhere [25,26].
AMD GRADING:
Grading was performed on the fundus images without further information on age, sex, risk factors, or previous diseases by a single experienced examiner. If several images were available, the best quality image was selected. The selection was based on the visibility and illumination of the macula. Fundus images with insufficient image quality were excluded. Grading was done according to the Rotterdam classification for AMD [13,15] (Table 1). This classification system divides the changes into 5 stages, with sub-stages starting with stage 0a for an inconspicuous macular finding up to stage 4a and 4b as late stages. Macular changes were also classified into early, intermediate, and late changes. In our study, early changes included stages 0b and 1b of the Rotterdam classification. Intermediate stages include 0c, 1a, 1b, 2a, 2b and 3. Late stages consist of geographic atrophy in stage 4a and choroidal neovascularization in stage 4b [15] (Table 1).
RISK FACTORS:
The following variables were included in this analysis: age, sex, ferritin, CRP, height, weight, hip circumference, waist circumference, physical activity, and diet. Obesity was defined as BMI≥30 m2/kg. Diet was surveyed based on the consumption of food in categories. These included sausage, meat, fruit, vegetables, and confectionery. Self-reported smoking was divided in current smoking, former smoking, and never smoking. Hypertension was defined as using antihypertensive medication, systolic blood pressure >140 mmHg, or diastolic blood pressure >90 mmHg. Cardiovascular diseases were defined as history of ischemic heart disease, myocardial infarction, or stroke. Dyslipidemia was defined by a low-density lipoprotein (LDL) to high-density lipoprotein (HDL) ratio (LDL/HDL) of >3.5, triglyceride levels >1.69 mmol/l, use of lipid-lowering medication, or diagnosis by a physician. Diabetes mellitus was defined by a diagnosis of a physician, known therapy (oral medication or insulin), or glycated hemoglobin (HbA1c)≥5%. Socioeconomic factors included family status, housing situation, number of people living in the house/flat, number of school years, school-leaving qualifications, training/studies, and equivalent household income (Table 2).
STATISTICAL ANALYSIS:
All available data were analyzed for accuracy and completeness. The prevalence for AMD stages was weighted for drop-out from SHIP-TREND-0 to SHIP-TREND-1 and for missing data due to non-gradable fundus images. To achieve these weights, logistic regression models with data availability yes/no as outcome and explanatory variables as independent variables were conducted. Participation at follow-up was explained by baseline variables from SHIP-TREND-0 and the missingness in SHIP-TREND-1 was explained by variables from SHIP-TREND-1. The inverse of the individual predictions from these models was calculated. The inverse probability weights were used multiplicatively in all analyses. Furthermore, we report characteristics of the study population in individuals with and without gradable fundus images. Continuous variables are reported as median and 25th and 75th percentiles, and differences among groups were tested by Mann-Whitney U tests. Categorical variables were reported as percentage and differences were tested by χ2 tests.
The AMD prevalence was determined for the total cohort as well as for subgroups after stratification by age and sex. We defined the following subgroups: (a) all AMD, (b) early AMD (drusen and/or PE changes), (c) intermediate AMD (drusen with PE changes), and (d) end-stage AMD (geographic atrophy or choroidal neovascularization).
Associations of potential risk factors with AMD prevalence were analyzed by logistic regression models adjusted for age in the total study population as well as stratified by sex (1 model for each potential risk factor). Fractional polynomials were tested to account for potential nonlinear associations between the potential risk factors and AMD prevalence [27]. The dose–response relationship was found using FP up to degree 2 with all possible combinations of powers selected from the set (−2, −1, −0.5, 0, 0.5, 1, 2, 3) and comparing them using the log likelihood to determine the best-fitting model. If none of the fractional polynomial models fitted the data significantly better than the linear model, linear regression was applied. In all analyses, the significance level was set at P<0.05. Statistical analysis was performed with Stata 17.0 (StataCorp LLC, Texas, USA).
Results
AMD GRADING:
We were able to include 1418 fundus images in this analysis. Small hard drusen were found in 3.88% of all subjects and soft drusen in 2.61%. Confluent drusen with pigment epithelial changes were found in 1.48% of the subjects and only pigment epithelial changes in 3.88%. Early forms of AMD according to the Rotterdam classification were thus detectable in 6.49% (0b with 2.61% (95% CI [1.84, 3.63]) and 1b with 3.88% (95% CI [2.40, 4.30]), Table 1, Figures 3, 4) of subjects. An intermediate AMD stage was identified in 3.88% (0c with 1.27% (95% CI [0.70, 1.86]), 1a with 0.99% (95% CI [0.38, 1.13]), 2a with 1.06% (95% CI [0.35, 1.00]), 2b with 0.14% (95% CI [0.02, 0.35]), 3 with 0.42% (95% CI [0.10, 0.55]), Table 1, Figures 3, 4) of all individuals. The prevalence of late AMD forms was 0.07% (4a with 0.07% (95% CI [0.01, 0.19]) and 4b with 0.00%, Table 1, Figures 3, 4). The only late form was geographic atrophy in 1 patient (Table 1, Figures 3, 4). Early changes in AMD were mainly observed in individuals aged 40–69 years. Intermediate stages occurred mainly in individuals aged 60–79 years. The only late stage showed up in the age group over 80 years (Figure 4).
The main limitation of this image-based AMD grading was that 1040 images were not gradable mainly due to insufficient image quality (Table 1, Figure 2). A comparison of the subjects with gradable and non-gradable fundus images is shown in Table 3.
RISK FACTORS:
We investigated associations of AMD-associated health variables with a combined AMD-variable including all stages. The AMD-associated health variables were age, sex, nicotine consumption, physical activity, obesity, somatometric parameters, cardiovascular risk factors, blood parameters such as HbA1c, total cholesterol, HDL cholesterol, LDL cholesterol and triglycerides, CRP, ferritin, TSH, fT3, fT4 (Table 2), and dietary patterns. We found a nonlinear positive association between age and AMD (P>0.001) (Figure 5A). The risk for AMD increased more strongly in individuals older than 70 years. In the further investigation, the number of school years was non-linearly associated for the total cohort (P=0.020) (Figure 5D) and positively associated in the sex-separated analysis for men (P=0.001) (Table 2). The lower the number of school years, the higher was the risk for AMD. Smoking showed a positive association with AMD in men but not in women (Table 2). A higher BMI was associated with a higher risk for AMD in men but not in women (Table 2). Nonlinear associations between HDL cholesterol and AMD were found in the total cohort (P=0.001) (Table 2, Figure 5B). The risk of AMD was increased at a value below 1.2 mmol/l. In the analysis of men and women separately, an association between low HDL cholesterol and AMD was found, especially in female subjects (P=0.004) (Table 2). In the further analyses we found an increased fT3 value (P=0.017) to be non-linearly associated with AMD (Table 2, Figure 5C). The risk of AMD increased with the fT3 level. In the separate analysis of men and women, a significant association was shown between AMD and an increased fT3 in women (P=0.039) (Table 2). Waist circumference, hip circumference, height, waist-to-weight ratio, waist-to-hip ratio, waist-to-weight ratio, HbA1c, diabetes, total cholesterol, LDL cholesterol, triglycerides, hypertension, CRP, ferritin, TSH, and fT4 showed no significant associations with AMD (Table 2). Using the Bonferroni correction, a P value of 0.0007 would still reveal statistically significant findings for age, but the associations of school years, HDL cholesterol, smoking, and fT3 in women would not be significant anymore.
Discussion
This paper describes the SHIP-TREND-1 cohort regarding AMD and adds further information on risk factors associated with AMD in northeast Germany.
AMD grading of the fundus images showed early changes in 6.49% of the subjects (stage 0b with 2.61% and stage 1b with 3.88%, Table 1, Figures 3, 4), intermediate stages were seen in 3.88% (stage 0c with 1.27%, stage 1a with 0.99%, stage 2a with 1.06%, stage 2b with 0.14%, stage 3 with 0.42%, Table 1, Figures 3, 4), and late stages were seen in 1 subject (stage 4a with 0.07%, Table 1, Figures 3, 4). In the risk factor analysis, the number of school years, elevated HDL cholesterol, and elevated fT3 were associated with AMD in the overall cohort. In the sex-dependent analysis, an increased BMI and smoking were found to be positively associated with AMD in men (Table 2).
The grading of SHIP-TREND-1 showed a comparable distribution of stages compared to the baseline examination. In our cohort, we found a higher frequency of early changes, especially retinal pigment epithelial changes, compared to other national and international studies. Late stages, however, hardly occurred. This is probably due to the high number of excluded subjects due to reduced image quality, since image quality tended to be lower in older subjects, leading to an overrepresentation of older subject (with higher risk for late AMD) in the group of excluded subjects. This was shown in SHIP-TREND-1 and SHIP-TREND-0. The analysis of risk factors in SHIP-TREND-1 revealed associations with various metabolic pathways and socioeconomic factors in addition to factors related to obesity, which were mainly associated as risk factors in SHIP-TREND-0.
The study sample of the Rotterdam Eye study included 6251 participants between 55 and 98 years of age. They reported an increase in the presence of drusen in subjects aged 55–64 years from 4.8% to 17.5% in subjects aged 85 years and older. Late stages of AMD were reported in 1.7% of all participants [15].
The Beaver Dam Eye Study in the USA included 4926 participants aged 43–86 years. There was an increase in large drusen in the age group of 43–54 years to subjects older than 75 years, from 1.7% to 24%, respectively. Pigment epithelial changes showed an increase between these age groups, from 7.3% to 26.6%. Late forms of AMD occurred in 0.1% of the 43–54 age group with exudative AMD. In subjects 75 years and older, wet AMD was found in 5.2% and geographic atrophy in 2.0% [16].
In the age group 49–54 years, the Blue Mountain Eye Study in Australia found signs of early AMD in 1.3% of subjects, but no signs of any stage of late AMD. There was a slight increase in early AMD stages with 2.6% in subjects 55–64 year of age and 0.2% had late stages of AMD. In the 65–74 age group, 8.5% of subjects had early changes and 0.7% had late stages [17].
In the 1418 fundus images available, there was a correlation between AMD and level of education. The fewer years of education, the higher the risk for any form of AMD (Figure 4), especially in men. Zou et al also showed a correlation between lower education level and AMD [28]. Similar results were found in the Hainan Study for all forms of AMD [29]. Cackett et al found an association with early forms of AMD [30]. It can therefore be assumed that a low educational level can be associated with a lower awareness of factors for health and illness. Low socioeconomic status is associated with various chronic diseases like coronary heart disease, stroke, and some cancers [9,31], especially in men [32]. These health problems are often linked to a poor diet. The diet of individuals with a low socioeconomic status tends to be higher in fat and low in nutrients such as sodium, magnesium, vitamin C, folic acid, and iron [31]. In the SHIP-TREND-0 and in SHIP-TREND-1 studies, no association between diet and AMD could be determined, presumably because it was not possible to identify clear dietary patterns. Nevertheless, there seems to be a connection. A diet rich in fruit and vegetables and fish shows an overall protective effect against AMD [33]. The AREDS study was also able to demonstrate a protective effect on the progression of early AMD for certain dietary supplements such as vitamins C and E, zinc, lutein, zeaxanthin, and omega-3 fatty acids [11,34]. These supplements are found in the Mediterranean diet with a high proportion of vegetables and fruit as well as fish. Several studies on the Mediterranean diet have shown a reduced risk of progression to late stages of AMD and reduced formation of large drusen [33–36]. These antioxidants lead to a reduction of oxidative stress and inflammatory reactions, which are important in the pathogenesis of AMD. Oxidative stress also plays a role in fat metabolism and in the regulation of lipoproteins. Our examination of blood lipid levels showed an association of AMD with lower HDL, which was, however, more common in women, but this was not found in the SHIP-TREND-0 study. Other studies have shown inconsistent results regarding AMD and lipid levels, but an association with HDL cholesterol was most commonly reported, while other lipoproteins were rarely identified [37]. A sex-dependent association of AMD in relation to the expression of lipoproteins was also reported [38]. Some studies have suggested that AMD progression in women follows other processes due to estrogen. Estrogen may have a protective effect and can lead to changes in serum lipids with higher HDL molecule levels and have antioxidant properties, although these disappear after menopause [38]. HDL in particular plays a central role through regulation of the complement system. Genetic studies showed the presence of complement factors and inflammatory proteins in HDL molecules [39]. In a metabolomics analysis, a total of 60 metabolites were found to be associated with AMD. In particular, an association was found between AMD and very high and high HDL as well as with low VLDL. Furthermore, a strong association of genetic variants in or next to genes involved in lipid metabolism was found. In further analysis, 57 of the 60 metabolites identified in association with AMD were significantly associated with the complement system. This again highlights the complexity of the pathogenesis and the need to develop multimodal therapeutic approaches [40].
Smoking is the most important modifiable risk factor for AMD and is a major trigger of oxidative stress and inflammation. Smoking leads to reduction of macular pigment, deterioration of choroidal blood flow, and reduction of protective serum antioxidants [41,42]. In our study, there was a positive association between smoking and AMD in men, while women showed an inverse relationship. This fact could not be clarified through our literature research or through a revision of our data and is not compatible with the previous general results on smoking as the most important risk factor for AMD. Regarding smoking as a risk of AMD, further investigations are needed in the follow-ups of SHIP-TREND-1. We currently assume a there is bias. In SHIP-TREND-0, smoking was not associated with AMD in men or women. Overall, over 30% of the subjects were younger than 40 years in the baseline cohort and only a few late forms of AMD were found, so the damage caused by smoking and oxidative stress was not yet clearly visible.
Another factor in relation to inflammation and lipoprotein levels is body fat. We found an association with AMD and high BMI only in men in our cohort. Similar results were also found in other studies. In the work of Seddon et al, a correlation between increased BMI and the progression to late AMD was demonstrated, and the relative risk increased with BMI [43]. In this context, an association between AMD and waist circumference, as a parameter related to abdominal fat, has also been reported [43]. BMI was also shown to be positively associated with AMD in SHIP-TREND-0, but for the entire cohort. The same applies to waist circumference in SHIP-TREND-0, which could not be proven as an association in SHIP-TREND-1. A closer look at the subjects revealed no obvious cause for this discrepancy, so there may be a selection bias due to the exclusion of 1040 subjects.
Furthermore, Graves’ disease is also associated with AMD due to an increase in oxidative metabolism and the increased formation of free oxygen radicals [44]. In this regard, the investigation is being carried out for the first time in SHIP-TREND-1. SHIP-TREND-0 did not collect any data on this for AMD. There is evidence that thyroid hormones can affect the retinal pigment epithelium [45], while TSH itself leads to photoreceptor damage [46]. In the Rotterdam Study, elevated fT4 was associated with the occurrence of AMD [45]. Similar results were obtained in the Blue Mountain Eye Study [47] and a case-control study by Hung et al [48]. The consideration of hyperthyroidism was related to TSH and fT4. In our study, however, only fT3 was found to be associated with AMD (Figure 5C). Thyroid function influences other metabolic processes, such as lipid metabolism. fT3 is more biologically active than the prohormone fT4 [49]. A cross-sectional study by Post et al showed a connection between increased fT3 values and increased HDL cholesterol particle concentration, but not directly with HDL cholesterol. It therefore supports our assumption that there could be an indirect connection with AMD via lipid metabolism. It is believed that the association between fT3 and circulating lipoproteins and lipids is more relevant than an association with fT4, and increased fT3 leads to increased synthesis of HDL particles [50,51]. Further investigations are needed to clarify the relationship of thyroid hormones with AMD.
Regarding blood pressure, HbA1c, CRP, and ferritin, there are inconsistent results in the literature. In particular, the connection with blood pressure as another cardiovascular risk factor alongside dyslipidemia and smoking could not always be reproduced as a risk factor for AMD, for example in the Beaver Dam Eye Study [37], while a meta-analysis by van Lookeren Campagne found a significant association between elevated blood pressure and AMD [52]. The situation is similar with HbA1c, which has been shown to be associated primarily with late forms of AMD [53]. Elevated CRP and decreased ferritin are also associated with AMD by promoting oxidative stress [8,54,55].
A main limitation of this study is the predominantly White population, which limits the ability to generalize our study results. To ensure comparability within the SHIP-TREND study and across Europe, the grading was carried out using the Rotterdam classification. Other international grading systems are slightly different, so there are minor deviations, particularly in the classification of early and intermediate stages. Another important limitation of this study is that a total of 1040 fundus images had to be excluded due to low image quality or unclear lesions. Pictures of older persons with corresponding risk factors were especially affected by this, so a selection bias exists and limits the generalizability of this study (Figure 4); turbidity of the refractive media (ie, the presence of a cataract) was a major cause of this. The exclusion of fundus photographs of older subjects due to low image quality probably means that not all late AMD cases could be identified and included. This shows that the risk factors discussed here should be classified in connection with early and intermediate forms of AMD and therefore also for younger individuals. In addition, out of consideration for other examinations, the pupil was not dilated and a non-mydriatic camera was therefore used. We assume that an examination under medical mydriasis would lead to higher image quality and more gradable images. The main strength of this study is the population-based design and the large number of subjects and variables investigated.
Conclusions
Our analysis of AMD risk factors showed an association with lipid and thyroid metabolism in the overall cohort and, particularly in men, with smoking and obesity. These factors and metabolic pathways are involved in the development of oxidative stress. There is a need to take the different metabolic pathways into account in patient care and the development of therapy concepts, especially for dry AMD. Due to the association with educational level, there is a possible connection with socioeconomic factors. Further investigations are needed in this regard.
Figures
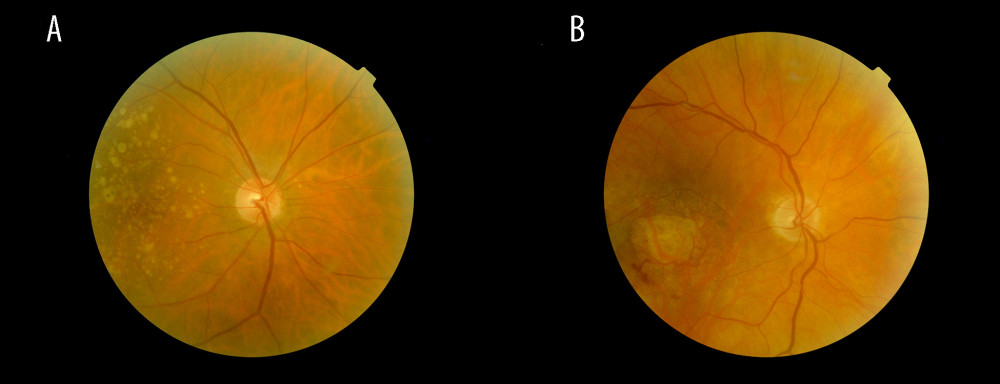 Figure 1. Fundus images of intermediate AMD (A) and late-stage geographic atrophy (B). Image A shows the typical AMD-associated changes in the form of drusen (ie, yellow deposits in the pigment epithelium), as well as pigment epithelium changes in the form of hyperpigmentation. Image B shows atrophy of the retinal pigment epithelium and photoreceptors in the central macula area.
Figure 1. Fundus images of intermediate AMD (A) and late-stage geographic atrophy (B). Image A shows the typical AMD-associated changes in the form of drusen (ie, yellow deposits in the pigment epithelium), as well as pigment epithelium changes in the form of hyperpigmentation. Image B shows atrophy of the retinal pigment epithelium and photoreceptors in the central macula area. 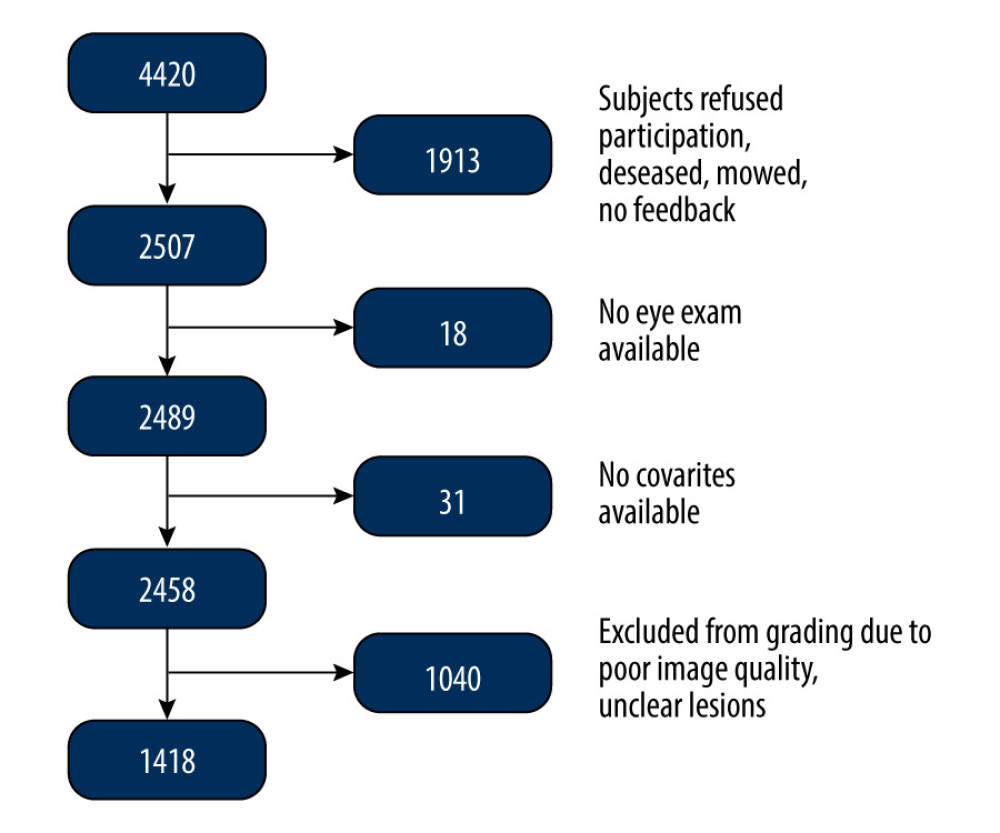 Figure 2. Flowchart of the SHIP-TREND-1 study population. Of the 4420 subjects examined in the SHIP-TREND baseline study, 2507 subjects were included in the first follow-up SHIP-TREND-1. Of these, 18 subjects did not receive an eye examination, so grading of the fundus image was performed in 2489 subjects. A further 31 subjects had to be excluded due to missing covariates and 1040 subjects due to low image quality or unclear retinal changes, so 1418 subjects could be included in the statistical analysis.
Figure 2. Flowchart of the SHIP-TREND-1 study population. Of the 4420 subjects examined in the SHIP-TREND baseline study, 2507 subjects were included in the first follow-up SHIP-TREND-1. Of these, 18 subjects did not receive an eye examination, so grading of the fundus image was performed in 2489 subjects. A further 31 subjects had to be excluded due to missing covariates and 1040 subjects due to low image quality or unclear retinal changes, so 1418 subjects could be included in the statistical analysis. 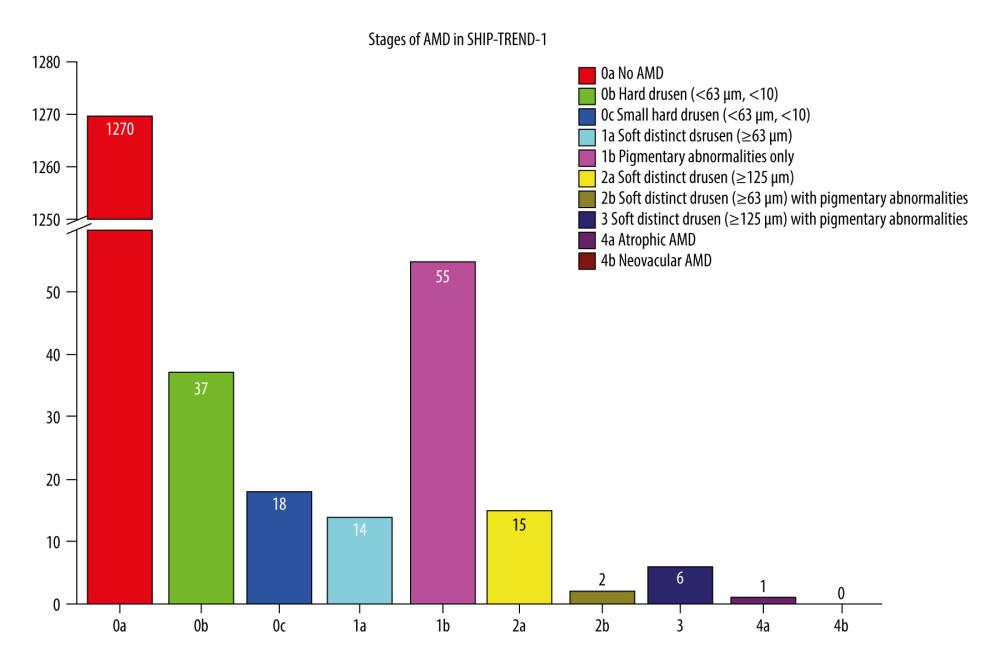 Figure 3. Frequency of age-related macular degeneration (AMD) stages in the SHIP-TREND-1 cohort. Most of the subjects included in the statistical analysis showed no abnormalities on fundus imaging. Only 55 subjects showed pigmentary changes in the macular area as part of the initial changes of AMD. There were only a few late AMD changes and only 1 final (dry) stage of AMD (stage 4a). This final stage, a geographic atrophy, is shown in Figure 1B. (Stata 17.0, StataCorp LLC, Texas, USA).
Figure 3. Frequency of age-related macular degeneration (AMD) stages in the SHIP-TREND-1 cohort. Most of the subjects included in the statistical analysis showed no abnormalities on fundus imaging. Only 55 subjects showed pigmentary changes in the macular area as part of the initial changes of AMD. There were only a few late AMD changes and only 1 final (dry) stage of AMD (stage 4a). This final stage, a geographic atrophy, is shown in Figure 1B. (Stata 17.0, StataCorp LLC, Texas, USA). 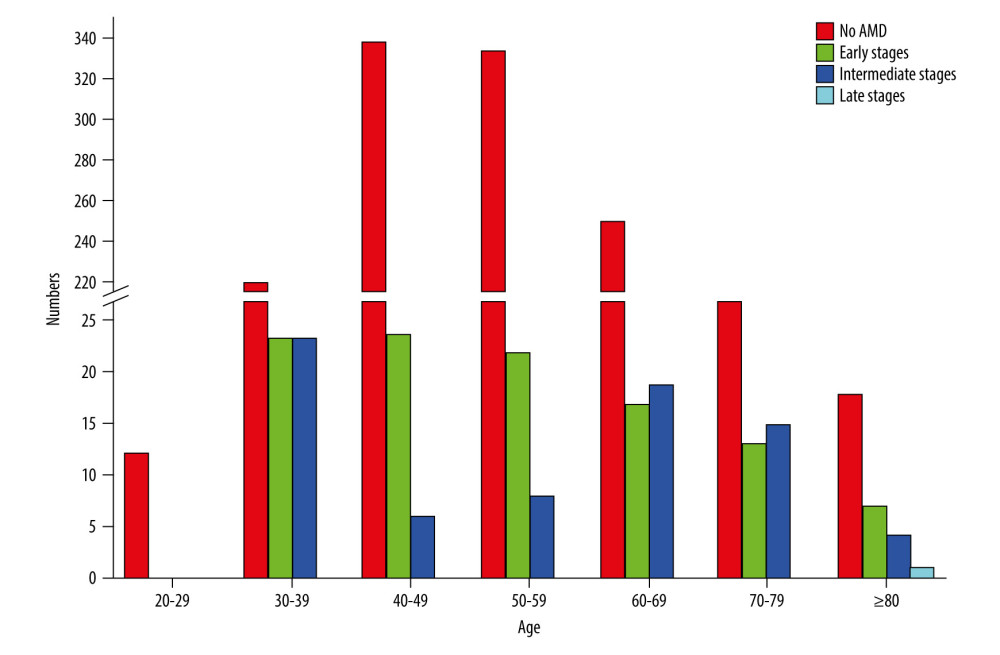 Figure 4. Frequency of age-related macular degeneration (AMD) stages by age groups in the SHIP-TREND-1 cohort. Most subjects included in this analysis were 40–60 years old. Although this is a rather young study population, the fact that many initial changes in the form of retinal pigment epithelial changes or small drusen were nevertheless detectable should be noted and shows the need for earlier examination. (Stata 17.0, StataCorp LLC, Texas, USA).
Figure 4. Frequency of age-related macular degeneration (AMD) stages by age groups in the SHIP-TREND-1 cohort. Most subjects included in this analysis were 40–60 years old. Although this is a rather young study population, the fact that many initial changes in the form of retinal pigment epithelial changes or small drusen were nevertheless detectable should be noted and shows the need for earlier examination. (Stata 17.0, StataCorp LLC, Texas, USA). 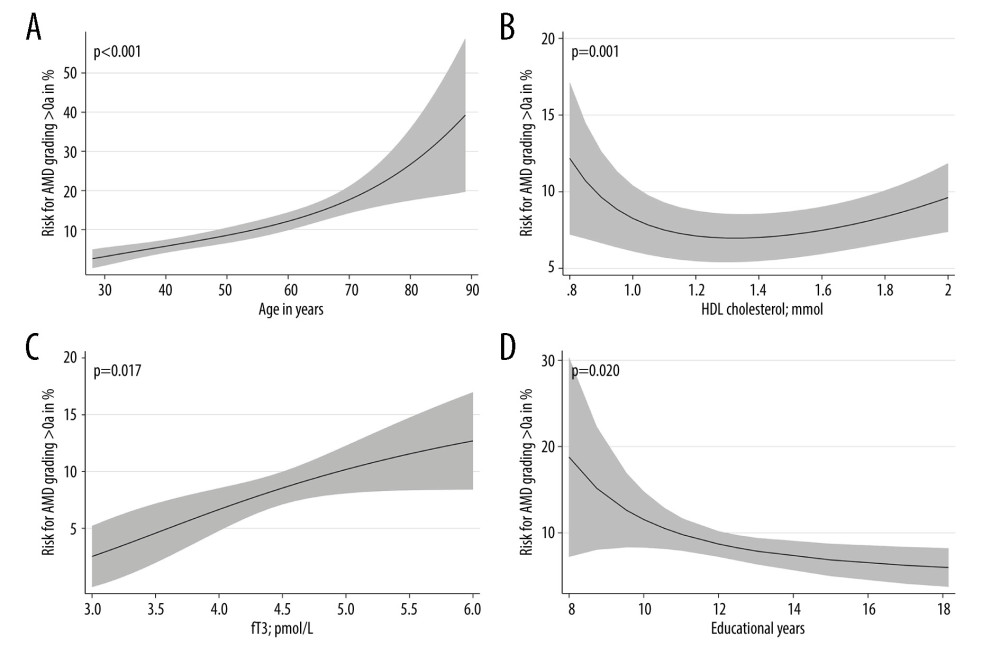 Figure 5. Nonlinear associations of age (A), HDL cholesterol (B), fT3 (C), and educational level (D) with AMD based on logistic regression models adjusted for age (for models with HDL cholesterol, fT3 or educational level). Nonlinear relations were detected systematically by fractional polynomials. (Stata 17.0, StataCorp LLC, Texas, USA).
Figure 5. Nonlinear associations of age (A), HDL cholesterol (B), fT3 (C), and educational level (D) with AMD based on logistic regression models adjusted for age (for models with HDL cholesterol, fT3 or educational level). Nonlinear relations were detected systematically by fractional polynomials. (Stata 17.0, StataCorp LLC, Texas, USA). References
1. VanDenLangenberg AM, Carson MP, Drusen bodies: StatPearls [Internet] May 1, 2023, Treasure Island (FL), StatPearls Publishing
2. Thomas CJ, Mirza RG, Gill MK, Age-related macular degeneration: Med Clin North Am, 2021; 105(3); 473-91
3. Ruia S, Kaufman EJ, Macular degeneration: StatPearls [Internet] Jul 31, 2023, Treasure Island (FL), StatPearls Publishing
4. Mitchell J, Bradley C, Quality of life in age-related macular degeneration: A review of the literature: Health Qual Life Outcomes, 2006; 4; 97
5. Mitchell P, Liew G, Gopinath B, Wong TY, Age-related macular degeneration: Lancet, 2018; 392(10153); 1147-59
6. Haines JL, Hauser MA, Schmidt S, Complement factor H variant increases the risk of age-related macular degeneration: Science, 2005; 308(5720); 419-21
7. Hsueh YJ, Chen YN, Tsao YT, The pathomechanism, antioxidant biomarkers, and treatment of oxidative stress-related eye diseases: Int J Mol Sci, 2022; 23(3); 1255
8. Datta S, Cano M, Ebrahimi K, The impact of oxidative stress and inflammation on RPE degeneration in non-neovascular AMD: Prog Retin Eye Res, 2017; 60; 201-18
9. Pennington KL, DeAngelis MM, Epidemiology of age-related macular degeneration (AMD): Associations with cardiovascular disease phenotypes and lipid factors: Eye Vis (Lond), 2016; 3; 34
10. Wang SB, Mitchell P, Chiha J, Severity of coronary artery disease is independently associated with the frequency of early age-related macular degeneration: Br J Ophthalmol, 2015; 99(3); 365-70
11. Sin HP, Liu DT, Lam DS, Lifestyle modification, nutritional and vitamins supplements for age-related macular degeneration: Acta Ophthalmol, 2013; 91(1); 6-11
12. Schultz NM, Bhardwaj S, Barclay C, Global burden of dry age-related macular degeneration: A targeted literature review: Clin Ther, 2021; 43(10); 1792-818
13. Schuster AK, Wolfram C, Pfeiffer N, Finger RPOphthalmology 2019 – where do we stand?: An analysis of the treatment situation in Germany: Ophthalmologe, 2019; 116(9); 829-37 [in German]
14. Wong WL, Su X, Li X, Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis: Lancet Glob Health, 2014; 2(2); e106-16
15. Vingerling JR, Dielemans I, Hofman A, The prevalence of age-related maculopathy in the Rotterdam Study: Ophthalmology, 1995; 102(2); 205-10
16. Klein R, Klein BEK, Linton KLP, Prevalence of age-related maculopathy: The Beaver Dam Eye Study: Ophthalmology, 2020; 127(4S); S122-S32
17. Mitchell P, Smith W, Attebo K, Wang JJ, Prevalence of age-related maculopathy in Australia. The Blue Mountains Eye Study: Ophthalmology, 1995; 102(10); 1450-60
18. Buch H, Nielsen NV, Vinding T, 14-year incidence, progression, and visual morbidity of age-related maculopathy: The Copenhagen City Eye Study: Ophthalmology, 2005; 112(5); 787-98
19. Li Y, Xu L, Wang YX, Prevalence of age-related maculopathy in the adult population in China: The Beijing Eye Study: Am J Ophthalmol, 2008; 146(2); 329
20. Kawasaki R, Wang JJ, Aung TSingapore Malay Eye Study Group, Prevalence of age-related macular degeneration in a Malay population: The Singapore Malay Eye Study: Ophthalmology, 2008; 115(10); 1735-41
21. Oshima Y, Ishibashi T, Murata T, Prevalence of age-related maculopathy in a representative Japanese population: The Hisayama Study: Br J Ophthalmol, 2001; 85(10); 1153-57
22. Bastawrous A, Mathenge W, Peto T, Six-year incidence and progression of age-related macular degeneration in Kenya: Nakuru Eye Disease Cohort Study: JAMA Ophthalmol, 2017; 135(6); 631-38
23. Lüdtke L, Jürgens C, Ittermann T, Age-related macular degeneration and associated risk factors in the population-based study of health in Pomerania (SHIP-Trend): Med Sci Monit, 2019; 25; 6383-90
24. Brandl C, Zimmermann ME, Günther F, On the impact of different approaches to classify age-related macular degeneration: Results from the German AugUR Study: Sci Rep, 2018; 8(1); 8675
25. Jürgens C, Völzke H, Tost FStudy of health in Pomerania (SHIP-Trend): Important aspects for healthcare research in ophthalmology: Ophthalmologe, 2014; 111(5); 443-47 [in German]
26. Völzke H, Schössow J, Schmidt CO, Cohort profile update: The Study of Health in Pomerania (SHIP): Int J Epidemiol, 2022; 51(6); e372-e83
27. Schmidt CO, Ittermann T, Schulz A, Linear, nonlinear or categorical: How to treat complex associations in regression analyses? Polynomial transformations and fractional polynomials: Int J Public Health, 2013; 58(1); 157-60
28. Zou M, Zhang Y, Chen A, Variations and trends in global disease burden of age-related macular degeneration: 1990–2017: Acta Ophthalmol, 2021; 99(3); e330-e35
29. Zhang K, Zhong Q, Chen S, An epidemiological investigation of age-related macular degeneration in aged population in China: The Hainan Study: Int Ophthalmol, 2018; 38(4); 1659-67
30. Cackett P, Tay WT, Aung T, Education, socio-economic status and age-related macular degeneration in Asians: The Singapore Malay Eye Study: Br J Ophthalmol, 2008; 92(10); 1312-15
31. James WP, Nelson M, Ralph A, Leather S, Socioeconomic determinants of health. The contribution of nutrition to inequalities in health: BMJ, 1997; 314(7093); 1545-49
32. O’Neil A, Scovelle AJ, Milner AJ, Kavanagh A, Gender/sex as a social determinant of cardiovascular risk: Circulation, 2018; 137(8); 854-64
33. Merle BMJ, Colijn JM, Cougnard-Grégoire AEYE-RISK Consortium, Mediterranean diet and incidence of advanced age-related macular degeneration: The EYE-RISK Consortium: Ophthalmology, 2019; 126(3); 381-90
34. Agrón E, Mares J, Clemons TEAREDS and AREDS2 Research Groups, Dietary nutrient intake and progression to late age-related macular degeneration in the age-related eye disease studies 1 and 2: Ophthalmology, 2021; 128(3); 425-42
35. Keenan TD, Agrón E, Mares JAge-Related Eye Disease Studies (AREDS) 1 and 2 Research Groups, Adherence to the mediterranean diet and progression to late age-related macular degeneration in the age-related eye disease studies 1 and 2: Ophthalmology, 2020; 127(11); 1515-28
36. Broadhead GK, Agrón E, Peprah DAREDS/AREDS2 Investigators, Association of dietary nitrate and a mediterranean diet with age-related macular degeneration among US adults: The Age-Related Eye Disease Study (AREDS) and AREDS2: JAMA Ophthalmol, 2023; 141(2); 130-39
37. Lin JB, Halawa OA, Husain D, Dyslipidemia in age-related macular degeneration: Eye (Lond), 2022; 36(2); 312-18
38. Heesterbeek TJ, Lorés-Motta L, Hoyng CB, Risk factors for progression of age-related macular degeneration: Ophthalmic Physiol Opt, 2020; 40(2); 140-70
39. Betzler BK, Rim TH, Sabanayagam C, High-density lipoprotein cholesterol in age-related ocular diseases: Biomolecules, 2020; 10(4); 645
40. Acar İE, Lores-Motta L, Colijn JMEYE-RISK Consortium, Integrating metabolomics, genomics, and disease pathways in age-related macular degeneration: The EYE-RISK Consortium: Ophthalmology, 2020; 127(12); 1693-709
41. Mullins RF, Russell SR, Anderson DH, Hageman GS, Drusen associated with aging and age-related macular degeneration contain proteins common to extracellular deposits associated with atherosclerosis, elastosis, amyloidosis, and dense deposit disease: FASEB J, 2000; 14(7); 835-46
42. Delcourt C, Delyfer MN, Rougier MB, Associations of complement factor H and smoking with early age-related macular degeneration: The ALIENOR Study: Invest Ophthalmol Vis Sci, 2011; 52(8); 5955-62
43. Seddon JM, Cote J, Davis N, Rosner B, Progression of age-related macular degeneration: association with body mass index, waist circumference, and waist-hip ratio: Arch Ophthalmol, 2003; 121(6); 785-92
44. Tsai CC, Kao SC, Cheng CY, Oxidative stress change by systemic corticosteroid treatment among patients having active graves ophthalmopathy: Arch Ophthalmol, 2007; 125(12); 1652-56
45. Chaker L, Buitendijk GH, Dehghan A, Thyroid function and age-related macular degeneration: A prospective population-based cohort study – The Rotterdam Study: BMC Med, 2015; 13; 94
46. Ma H, Thapa A, Morris L, Suppressing thyroid hormone signaling preserves cone photoreceptors in mouse models of retinal degeneration: Proc Natl Acad Sci USA, 2014; 111(9); 3602-7
47. Gopinath B, Liew G, Kifley A, Mitchell P, Thyroid dysfunction and ten-year incidence of age-related macular degeneration: Invest Ophthalmol Vis Sci, 2016; 57(13); 5273-77
48. Hung SH, Xirasagar S, Kuang TT, Association of age-related macular degeneration with prior hyperthyroidism and hypothyroidism: A case-control study: J Pers Med, 2022; 12(4); 602
49. Abdalla SM, Bianco AC, Defending plasma T3 is a biological priority: Clin Endocrinol (Oxf), 2014; 81(5); 633-41
50. Post A, Garcia E, Gruppen EG, Higher free triiodothyronine is associated with higher HDL particle concentration and smaller HDL particle size: J Clin Endocrinol Metab, 2022; 107(5); e1807-e15
51. Corsetti JP, Bakker SJ, Sparks CE, Dullaart RP, Apolipoprotein A-II influences apolipoprotein E-linked cardiovascular disease risk in women with high levels of HDL cholesterol and C-reactive protein: PLoS One, 2012; 7(6); e39110
52. van Lookeren Campagne M, LeCouter J, Mechanisms of age-related macular degeneration and therapeutic opportunities: J Pathol, 2014; 232(2); 151-64
53. Chen X, Rong SS, Xu Q, Diabetes mellitus and risk of age-related macular degeneration: a systematic review and meta-analysis: PLoS One, 2014; 9(9); e108196
54. Hong T, Tan AG, Mitchell P, Wang JJ, A review and meta-analysis of the association between C-reactive protein and age-related macular degeneration: Surv Ophthalmol, 2011; 56(3); 184-94
55. Čolak E, Žorić L, Radosavljević A, Ignjatović S, The association of serum iron-binding proteins and the antioxidant parameter levels in age-related macular degeneration: Curr Eye Res, 2018; 43(5); 659-65 Erratum in: Curr Eye Res. 2018;43(5):X
Figures
 Figure 1. Fundus images of intermediate AMD (A) and late-stage geographic atrophy (B). Image A shows the typical AMD-associated changes in the form of drusen (ie, yellow deposits in the pigment epithelium), as well as pigment epithelium changes in the form of hyperpigmentation. Image B shows atrophy of the retinal pigment epithelium and photoreceptors in the central macula area.
Figure 1. Fundus images of intermediate AMD (A) and late-stage geographic atrophy (B). Image A shows the typical AMD-associated changes in the form of drusen (ie, yellow deposits in the pigment epithelium), as well as pigment epithelium changes in the form of hyperpigmentation. Image B shows atrophy of the retinal pigment epithelium and photoreceptors in the central macula area. Figure 2. Flowchart of the SHIP-TREND-1 study population. Of the 4420 subjects examined in the SHIP-TREND baseline study, 2507 subjects were included in the first follow-up SHIP-TREND-1. Of these, 18 subjects did not receive an eye examination, so grading of the fundus image was performed in 2489 subjects. A further 31 subjects had to be excluded due to missing covariates and 1040 subjects due to low image quality or unclear retinal changes, so 1418 subjects could be included in the statistical analysis.
Figure 2. Flowchart of the SHIP-TREND-1 study population. Of the 4420 subjects examined in the SHIP-TREND baseline study, 2507 subjects were included in the first follow-up SHIP-TREND-1. Of these, 18 subjects did not receive an eye examination, so grading of the fundus image was performed in 2489 subjects. A further 31 subjects had to be excluded due to missing covariates and 1040 subjects due to low image quality or unclear retinal changes, so 1418 subjects could be included in the statistical analysis. Figure 3. Frequency of age-related macular degeneration (AMD) stages in the SHIP-TREND-1 cohort. Most of the subjects included in the statistical analysis showed no abnormalities on fundus imaging. Only 55 subjects showed pigmentary changes in the macular area as part of the initial changes of AMD. There were only a few late AMD changes and only 1 final (dry) stage of AMD (stage 4a). This final stage, a geographic atrophy, is shown in Figure 1B. (Stata 17.0, StataCorp LLC, Texas, USA).
Figure 3. Frequency of age-related macular degeneration (AMD) stages in the SHIP-TREND-1 cohort. Most of the subjects included in the statistical analysis showed no abnormalities on fundus imaging. Only 55 subjects showed pigmentary changes in the macular area as part of the initial changes of AMD. There were only a few late AMD changes and only 1 final (dry) stage of AMD (stage 4a). This final stage, a geographic atrophy, is shown in Figure 1B. (Stata 17.0, StataCorp LLC, Texas, USA). Figure 4. Frequency of age-related macular degeneration (AMD) stages by age groups in the SHIP-TREND-1 cohort. Most subjects included in this analysis were 40–60 years old. Although this is a rather young study population, the fact that many initial changes in the form of retinal pigment epithelial changes or small drusen were nevertheless detectable should be noted and shows the need for earlier examination. (Stata 17.0, StataCorp LLC, Texas, USA).
Figure 4. Frequency of age-related macular degeneration (AMD) stages by age groups in the SHIP-TREND-1 cohort. Most subjects included in this analysis were 40–60 years old. Although this is a rather young study population, the fact that many initial changes in the form of retinal pigment epithelial changes or small drusen were nevertheless detectable should be noted and shows the need for earlier examination. (Stata 17.0, StataCorp LLC, Texas, USA). Figure 5. Nonlinear associations of age (A), HDL cholesterol (B), fT3 (C), and educational level (D) with AMD based on logistic regression models adjusted for age (for models with HDL cholesterol, fT3 or educational level). Nonlinear relations were detected systematically by fractional polynomials. (Stata 17.0, StataCorp LLC, Texas, USA).
Figure 5. Nonlinear associations of age (A), HDL cholesterol (B), fT3 (C), and educational level (D) with AMD based on logistic regression models adjusted for age (for models with HDL cholesterol, fT3 or educational level). Nonlinear relations were detected systematically by fractional polynomials. (Stata 17.0, StataCorp LLC, Texas, USA). Tables
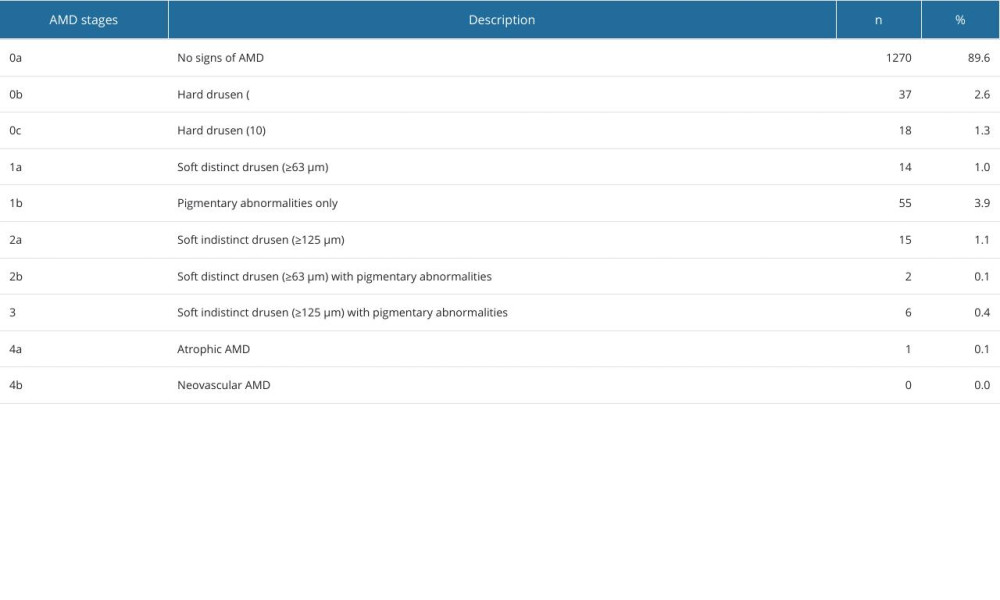 Table 1. Distribution of AMD stages according to the Rotterdam classification in SHIP-TREND-1.
Table 1. Distribution of AMD stages according to the Rotterdam classification in SHIP-TREND-1.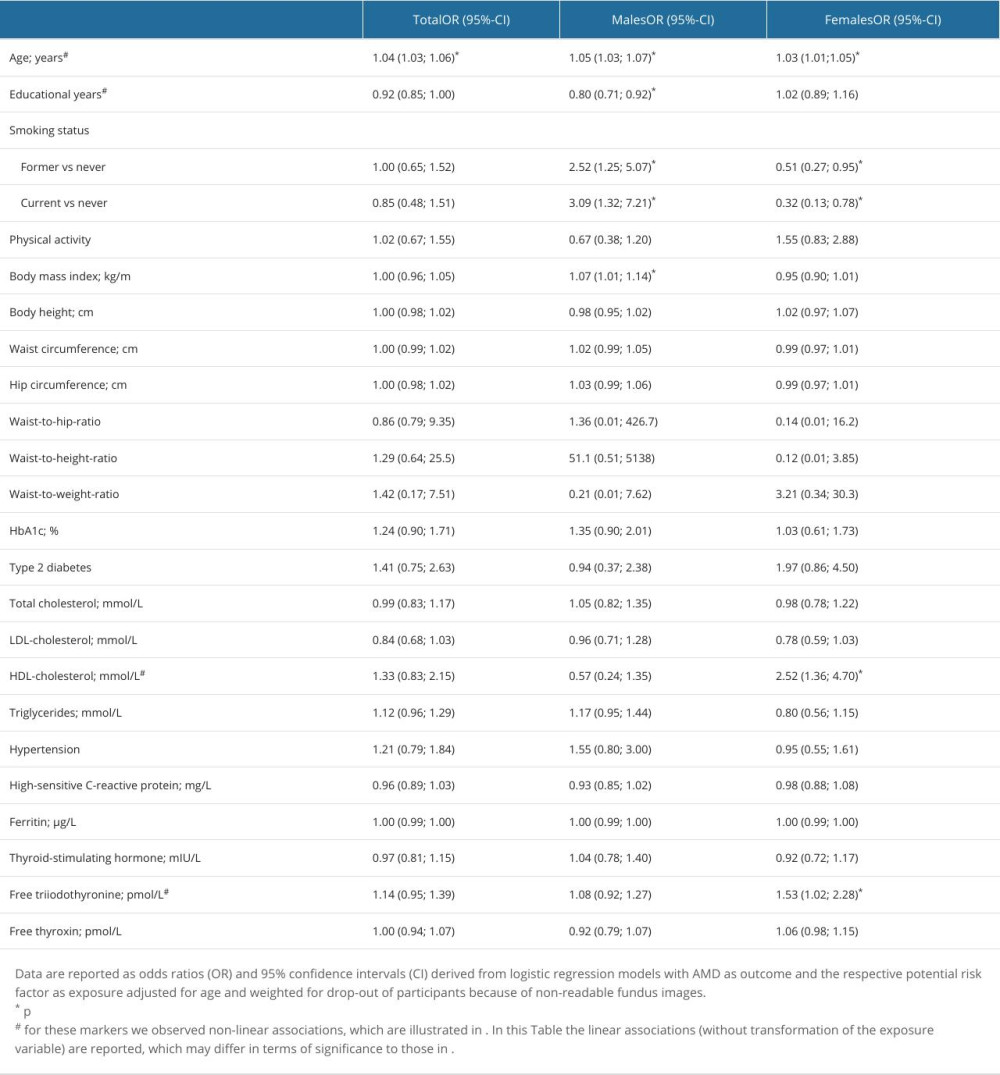 Table 2. Association of potential risk factors with AMD.
Table 2. Association of potential risk factors with AMD.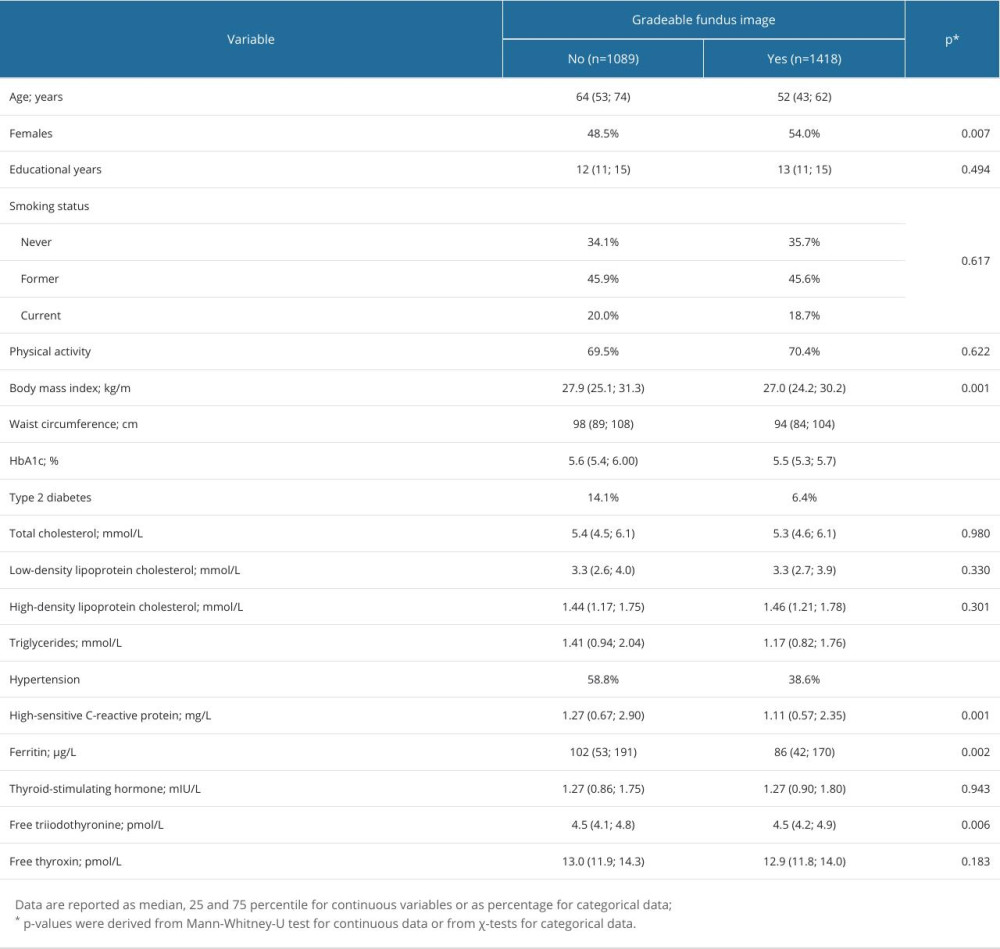 Table 3. Comparison of subjects with and without gradable fundus images in the SHIP-TREND-1 cohort.
Table 3. Comparison of subjects with and without gradable fundus images in the SHIP-TREND-1 cohort. Table 1. Distribution of AMD stages according to the Rotterdam classification in SHIP-TREND-1.
Table 1. Distribution of AMD stages according to the Rotterdam classification in SHIP-TREND-1. Table 2. Association of potential risk factors with AMD.
Table 2. Association of potential risk factors with AMD. Table 3. Comparison of subjects with and without gradable fundus images in the SHIP-TREND-1 cohort.
Table 3. Comparison of subjects with and without gradable fundus images in the SHIP-TREND-1 cohort. In Press
26 Mar 2024 : Clinical Research
New Computerized Planning Algorithm and Clinical Testing of Optimized Nuss Bar Design for Patients with Pec...Med Sci Monit In Press; DOI: 10.12659/MSM.943705
07 May 2024 : Clinical Research
Treatment of AVN-Induced Proximal Pole Scaphoid Nonunion Using a Fifth and Fourth Extensor Compartmental Ar...Med Sci Monit In Press; DOI: 10.12659/MSM.944553
16 Mar 2024 : Clinical Research
Diagnostic Efficiency of ACR-TIRADS Score for Differentiating Benign and Malignant Thyroid Nodules of Vario...Med Sci Monit In Press; DOI: 10.12659/MSM.943228
08 May 2024 : Clinical Research
Effect of Individualized PEEP Guided by Driving Pressure on Diaphragm Function in Patients Undergoing Lapar...Med Sci Monit In Press; DOI: 10.12659/MSM.944022
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








