15 April 2024: Clinical Research
Evaluation of the Interrater Reliability of Sonographic Measurements of Muscle Thickness of 38 Piriformis Muscles in 19 Patients with Piriformis Syndrome
Sibel Caglar1ABDEG*, Ozden Ozyemisci-Taskiran2ACDEGDOI: 10.12659/MSM.943720
Med Sci Monit 2024; 30:e943720
Abstract
BACKGROUND: The piriformis muscle is a flat superficial muscle of the deep gluteal muscles that externally rotates the hip. Ultrasound is widely used to identify the piriformis muscle, especially for guidance of the needle during injections; however, its diagnostic use has recently gained popularity. The operator-dependent nature of ultrasound requires demonstration of reliability between operators. This study aimed to evaluate interrater reliability of sonographic measurements of muscle thickness of 38 piriformis muscles in 19 patients with piriformis syndrome.
MATERIAL AND METHODS: An ultrasound transducer was placed transversely on the sacral spinous process and moved caudo-laterally until the piriformis muscle was visualized under the gluteus maximus while patients were lying in prone position. The thickness of piriformis muscle was measured with a 2 to 5-MHz broadband curvilinear transducer in 3 regions (thickest regions of muscle over the ilium, near the greater trochanter, and near the sacrum). The interrater reliability of measurements of 2 examiners who were blinded to each other’s measurements was assessed by intraclass correlation coefficient.
RESULTS: In total, 114 samples from 38 piriformis muscles of 19 patients with a diagnosis of piriformis syndrome were evaluated by 2 raters in this study. The median (interquartile range) patient age was 41 (15) years. Intraclass correlation coefficient value for overall thickness measurements of piriformis muscle was 0.836. Intraclass correlation coefficient values for 3 different regions were over the ilium, near the greater trochanter, and near the sacrum were 0.777, 0.883, and 0.811, respectively.
CONCLUSIONS: Ultrasound measurement of piriformis muscle thickness has good interrater reliability.
Keywords: Piriformis Muscle Syndrome, Reproducibility of Results, Diagnostic Imaging, Ultrasonography
Introduction
The piriformis muscle is a flat, pear-shaped external rotator of the hip, located deep in the gluteal region. The sciatic nerve exits the pelvis distal to this muscle except for some anatomical variations where the entire sciatic nerve or a division of it pass through or is proximal to the piriformis muscle [1]. The piriformis muscle has gained clinical significance in deep gluteal or extra-spinal sciatica pain syndrome owing to its close proximity to the sciatic nerve [2,3].
The piriformis muscle is not the sole muscle playing a role in deep gluteal pain [4], and “deep gluteal syndrome” is a more appropriate term to explain this clinical entity [5]; however, the term “piriformis syndrome” is still widely used today. This clinical condition is a constellation of symptoms, including buttock pain that can radiate down to the leg, and can be accompanied by sensory symptoms in the leg [3,6].
An irritated or inflamed piriformis muscle leading to the irritation of the sciatica nerve anterior to the piriformis muscle is thought to cause buttock and radiating leg pain in piriformis syndrome [6]. On the other hand, a hypertrophic, tight, and tender piriformis muscle might be responsible for the myofascial pain radiating down the leg without sciatic nerve compression [7]. On physical examination, palpation over the piriformis muscle and several specific maneuvers, such as flexion, adduction, internal rotation (FAIR), and pace (resisted abduction and external rotation of the thigh), provoke pain [6]. Diagnosis depends mainly on symptoms and clinical findings. Studies regarding radiological data showing changes in the piriformis muscle are evolving [7–9].
A hypertrophic piriformis muscle can be observed on magnetic resonance imaging (MRI) [3,8]. MRI and/or computerized tomography (CT) imaging exhibited pathologies related to the piriformis muscle in 64% of 116 patients with clinically suspected piriformis syndrome, and piriformis muscle enlargement was the most common imaging finding [9]. The authors suggest that cross sectional imaging of the piriformis muscle can alter treatment planning. Their study population mainly consisted of patients with trauma, infarction, infection, tumor, or other space-occupying lesions, which may explain the high rate of abnormal imaging findings.
Ultrasound is widely used in the identification of the piriformis muscle, especially for guidance of the needle during injections [10], and the diagnostic role of ultrasound has recently gained popularity in the research field [11–16]. On ultrasound, thickness of the piriformis muscle was measured as greater in patients with piriformis syndrome, by 0.4 cm and 0.5 cm, than the asymptomatic side of the same patient and healthy people, respectively [12,14]. Its low cost, easy accessibility, and absence of ionizing radiation enhances the value of ultrasound. However, its highly operator-dependent nature requires demonstration of reliability between different operators [10]. Present studies have not investigated reliability. Therefore, in this study, we aimed to evaluate the interrater reliability of sonographic measurements of piriformis muscle thickness in patients with piriformis syndrome.
Material and Methods
PARTICIPANTS:
Nineteen patients (15 women, 4 men) diagnosed with piriformis syndrome were included in the study. The inclusion criteria were patients aged >18 years with buttock pain and positive physical examination findings of piriformis syndrome, including tenderness upon palpation over the piriformis muscle, a positive FAIR test, and a positive pace maneuver [3,6,7]. Exclusion criteria were radiculopathy, low back pain, history of lower extremity or pelvic surgery, inability to walk, malignancy, and rheumatologic or neurologic disorders. Age, sex, height, and weight of all patients were recorded, and body mass index was calculated.
DATA COLLECTION:
A Hitachi Noblus (Hitachi Aloka Medical America, Inc) ultrasonic imaging system was used, with a 2 to 5-MHz broadband curvilinear (60 mm radiant) transducer. Device settings regarding image depth and gain were set according to the thickness of the overlying tissues of each patient to accurately visualize the deeply located piriformis muscle.
Participants were asked to lie in the prone position on the examination table with the gluteal regions undressed. Lower extremities were in the neutral position. The curvilinear transducer was placed directly onto the skin using sufficient transmission gel. The scanning protocol was started with placement of the ultrasound transducer transversely over the midline on the sacral spinous process, which was moved laterally to the posterior superior iliac spine and sacroiliac joint of the imaged side. Then, the transducer was moved caudally toward the greater sciatic foramen [17]. After visualizing the piriformis muscle under the gluteus maximus, the piriformis muscle was confirmed by demonstrating it got stretched with passive internal rotation of the hip with the knee flexed in 90 degrees [16]. Then, the lateral end of the transducer was slightly rotated along the longitudinal axis of the piriformis muscle (Figure 1). Three measurements of piriformis muscle thickness were performed: thickest parts of piriformis muscle over the ilium, on the lateral side of the ilium, near the greater trochanter, and on the medial side of the ilium, near the sacrum (Figure 2). Muscle thicknesses on longitudinal images were measured via the “distance” measurement function of the device. Measurements were performed in both extremities while the hip joint was in a neutral position.
INTERRATER RELIABILITY:
Two physiatrists, with intermediate to advanced musculoskeletal ultrasound skills, performed the ultrasound measurements. Prior to the study, the examiners conducted a pilot ultrasound study on 4 volunteers to standardize the scanning planes and measurement sites.
For interrater reliability, one of the examiners performed the ultrasound evaluation after the other on the same day. The examiners were blinded to each other’s examinations and measurements.
STATISTICAL ANALYSIS:
The power analysis was calculated using the online sample size tool [18] at the 95% power level and 5% statistical significance level. Under the assumptions of minimum acceptable reliability (ρ0) of 0.70 and expected reliability (ρ1) of 0.84 with 2 raters (k), it was calculated that 105 samples would be sufficient. In our study, each rater measured a total of 114 samples (3 measurements for each muscle, summing 3×38 measurements), reaching this sample size.
SPSS version 22.0 for Windows (IBM Corp, Armonk, NY, USA) was used for statistical analysis. The normality of the data distribution was analyzed using the Shapiro-Wilk test. Continuous variables with a normal distribution are presented as mean±standard deviation and continuous variables that were not normally distributed are presented as median (interquartile range). Categorical variables are presented as numbers. To compare muscle thickness measurements between the symptomatic and normal sides, the Wilcoxon signed-rank test was used. The interrater reliability of ultrasound measurements was assessed by the intraclass correlation coefficient analysis with the absolute agreement definition and 2-way mixed-effects model. Intraclass correlation coefficient values below 0.5, from 0.5 to 0.74, 0.75 to 0.89, and greater than 0.90 were defined as poor, moderate, good, and excellent reliability, respectively [19].
Results
DEMOGRAPHIC CHARACTERISTICS:
In total, 114 samples from 38 piriformis muscles of 19 patients were evaluated by 2 raters in this study. The median (interquartile range) age was 41 (15) years (min-max, 29–79). Mean height, weight, and body mass index values were 1.65±0.06 m (min–max, 1.50–1.80), 66.9±9.5 kg (min–max, 51–92) and 24.68±3.17 kg/m2 (min–max, 18.73–32.21), respectively. Two patients were left-handed.
Piriformis muscle thickness measurements of each examiner for dominant and non-dominant sides are given in Table 1. The intraclass correlation coefficient value for overall thickness measurements of piriformis muscle was 0.836. The intraclass correlation coefficient values in 3 regions of piriformis muscle over the ilium, near the greater trochanter, and near the sacrum were 0.777, 0.883, and 0.811, respectively (Table 2).
When the data were separated as symptomatic and asymptomatic side, there were no differences in the mean values of muscle thickness between the symptomatic and normal sides at 3 measurement sites (Table 3). The intraclass correlation coefficient value for overall thickness measurements of piriformis muscle was 0.877 (95% CI: 0.706–0.949, P<0.001) and 0.896 (95% CI: 0.616–0.968, P<0.001), for symptomatic and asymptomatic sides, respectively. Individual values for muscle thickness at 3 different points of piriformis in the symptomatic and normal side measured by examiner 1 are presented in Figures 3 and 4, respectively.
Discussion
This study demonstrated that ultrasound measurement of piriformis muscle thickness had good reliability when 2 different experienced examiners performed the measurements. The interrater reliability is clinically important during the diagnosis and follow-up of patients in both daily practice and research studies. Recent studies suggested morphological changes such as increased thickness [10–15], enlarged cross sectional area [11], change in echo-intensity [11,12] and elastography strain ratio [15,16] of the piriformis muscle or increased diameter of the sciatic nerve [12] in the symptomatic side of patients with piriformis syndrome. However, none of the studies investigated interrater reliability.
Piriformis muscle thickness measured by ultrasound is suggested to be able to discriminate between the symptomatic and asymptomatic sides in piriformis syndrome, with different cut-off values in different studies, such as 18.15 mm (sensitivity, 72%; specificity, 74%) [12], 17.85 mm (sensitivity, 60%; specificity, 95%) [16], and 9.95 mm (sensitivity, 95%; specificity, 88%) [13]. Two studies suggested similar values nearly twice that of the other study, leading to questions about the use of an absolute value for a cut-off point.
Measurement sites and positions differ among these studies. Siahaan et al measured thickness from the medial part of the tip of ilium parallel to the longitudinal plane at the sciatic notch while the leg of the patient was in 45-degree abduction [13]. This region is somewhat similar to over ilium measurement in our study; however, the position of the patient’s leg was neutral during our measurement. Other studies perform the measurement from the thickest portion of the muscle [12,14]. This corresponds most probably to the near sacrum site in our study. However, as shown in Figures 3 and 4, the thickest site of the piriformis might be any of the 3 measurement sites. This finding might be explained by the various shapes of the piriformis muscle. Since we demonstrated that overall measurement sites are reliable, any site can be used to measure thickness. However, to compare different measurements, it is essential to note the location of measurement in the records.
A scanning protocol for the piriformis muscle dynamically evaluating and confirming its visualization in healthy participants was defined by Battaglia et al, and was similar to our scanning protocol [17]. Passive internal and external rotation of the hip during scanning helps to distinguish the borders of the deeply located external rotators from the surrounding muscles. In that study, 2 ultrasonographers rated the diagnostic value of dynamic ultrasound in the pathologies of piriformis muscle as “likely”, but they neither performed a reliability study nor measured the thickness of the muscle. Broadhurst et al also investigated the morphology of the piriformis muscle by ultrasound, but only qualitatively [20]. Among a total of 27 patients with buttock pain, they found enlarged muscle in 15 patients, scarred and fibrotic muscle in 3 patients, and normal morphology in 9 patients. However, they did not clearly explain their scanning technique and did not make any quantitative measurement.
MRI was used to measure the cross-sectional area of the piriformis muscle by Grimaldi et al in advanced degenerative hip disease. They reported smaller muscle volumes in the degenerated leg by 14% and high intrarater reliability (intraclass correlation coefficient: 0.985) [21]. In low back pain with leg pain, piriformis muscle atrophy was detected on the symptomatic side on MRI [22]. Muscle volume measurements were performed by 3-dimensional manual segmentation of MR images and they reported high inter-rater reliability. This technique is highly costly and time-consuming. Eastlack et al [23] studied the intra- and interrater reliability of piriformis muscle assessment in axial T1-weighted images in 134 patients with pelvic dysfunction and back or buttock pain. They found that intra- and interrater reliabilities were moderate to good (kappa values were 0.39–0.60 and 0.74–0.82 for inter- and intraobserver, respectively). They categorized piriformis muscle asymmetry qualitatively. Approximately one-third of the patients had piriformis asymmetry, with hypertrophy more common than atrophy.
Russell et al [24] measured the thickness of the piriformis muscle in T1-weighted MRI in 100 patients, 77% of whom had back or buttock pain. They measured from only a single site (approximately 1–2 cm inferior to the sacroiliac joint), and the mean of their measurements was 1.9 cm, ranging from 0.8 to 3.2 cm. They measured the depth of the piriformis muscle from its posterior to anterior border in axial images. However, in the present ultrasonographic study, we measured the thickness of the piriformis muscle from its superior to inferior border. Our measurements could be compared with the values measured in coronal MR images.
In a MR neurography imaging study, Filler et al measured the piriformis muscle preoperatively in nearly 50 patients with piriformis syndrome, who had good to excellent outcomes after piriformis surgery. They observed ipsilateral piriformis muscle hypertrophy in 38.5% and atrophy in 15% in preoperative MR images [25]. Muscle asymmetry alone had lower specificity (66%) and sensitivity (46%) values in identifying piriformis syndrome than did including the asymmetry observed in hyperintensity of the sciatic nerve (specificity and sensitivity, 93% and 64%, respectively).
Haladaj et al evaluated anthropometric features of the piriformis muscle and sciatic nerve in 30 limbs of cadavers and reported the thickness of piriformis muscle as 31±6 mm in cadavers with normal muscle morphology [26]. They did not specify the location where they measured the thickness of piriformis muscle.
Park et al measured piriformis muscle thickness at 3 sites at the midsciatic notch level with CT scanning in 60 patients with no evidence of piriformis syndrome [27]. This site corresponds to the proximal part of the muscle that we measured near the sacrum in our study; however, they measured the depth of the piriformis muscle, similar to the MRI study by Russell et al.
Measurements by MRI or CT imaging mainly involve the depth size of the piriformis muscle in a single region. Thickness can be measured from coronal images. Ionizing radiation, low accessibility, and high economic cost are the disadvantages for these imaging studies. Since MRI is not an examiner-dependent technique, with excellent soft tissue resolution, it might serve as a comparison in validity studies of ultrasound. However, none of the recent studies mentioned above compared the ultrasound findings with that of MRI. One study measured the thickness and cross-sectional area of piriformis muscle by both ultrasound and MRI; however, ultrasonographic measurement results were not validated with MRI measurements [10].
One of the limitations of the present study is that intrarater reliability was not performed. This was not included in our study design not to invite the participants to the hospital in another day. Another limitation is that we did not perform a sample size analysis for comparing muscle thickness measurements between symptomatic and normal sides; therefore, our sample size is small for interpreting our findings in relation to the relevant literature.
The strength of this study is that this is the first interrater reliability study for measuring piriformis muscle thickness. The piriformis muscle was measured from 3 different sites, different from other studies.
Conclusions
Ultrasound measurement of piriformis muscle thickness was shown to have a good interrater reliability between 2 physiatrists who are experienced in musculoskeletal ultrasound.
Figures
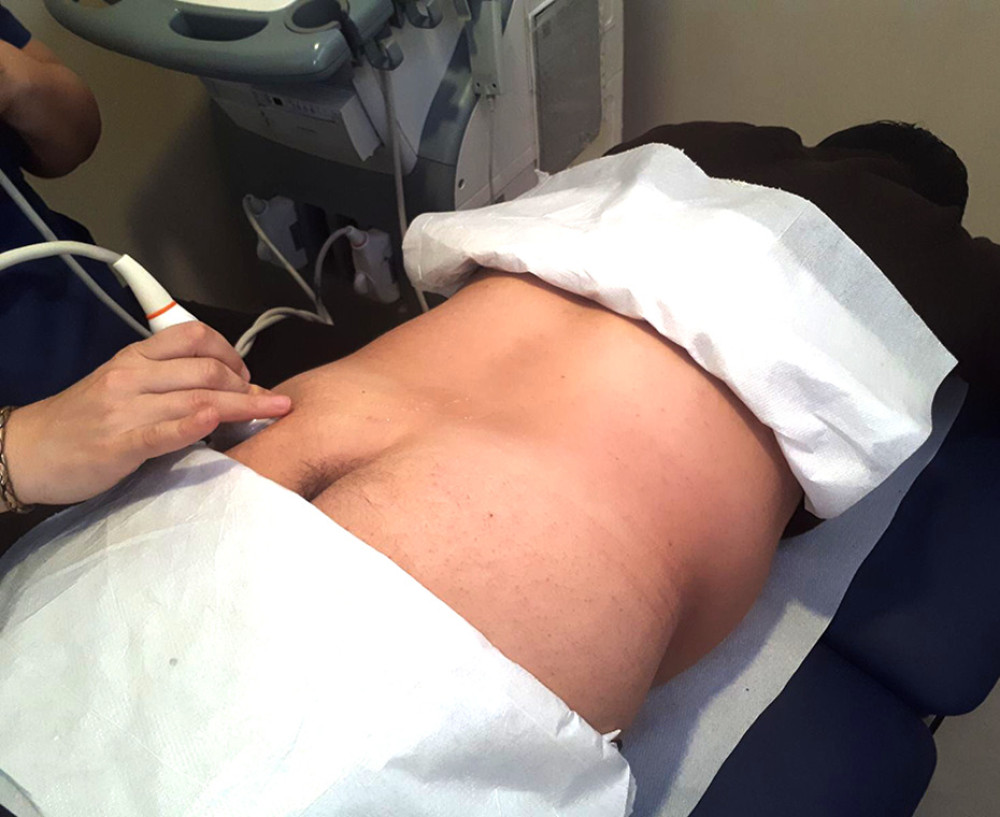 Figure 1. Placement of the ultrasound transducer over the piriformis muscle. The patient is lying in prone position with lower extremity in neutral position. Thickness measurement of piriformis muscle is performed in the longitudinal view.
Figure 1. Placement of the ultrasound transducer over the piriformis muscle. The patient is lying in prone position with lower extremity in neutral position. Thickness measurement of piriformis muscle is performed in the longitudinal view. 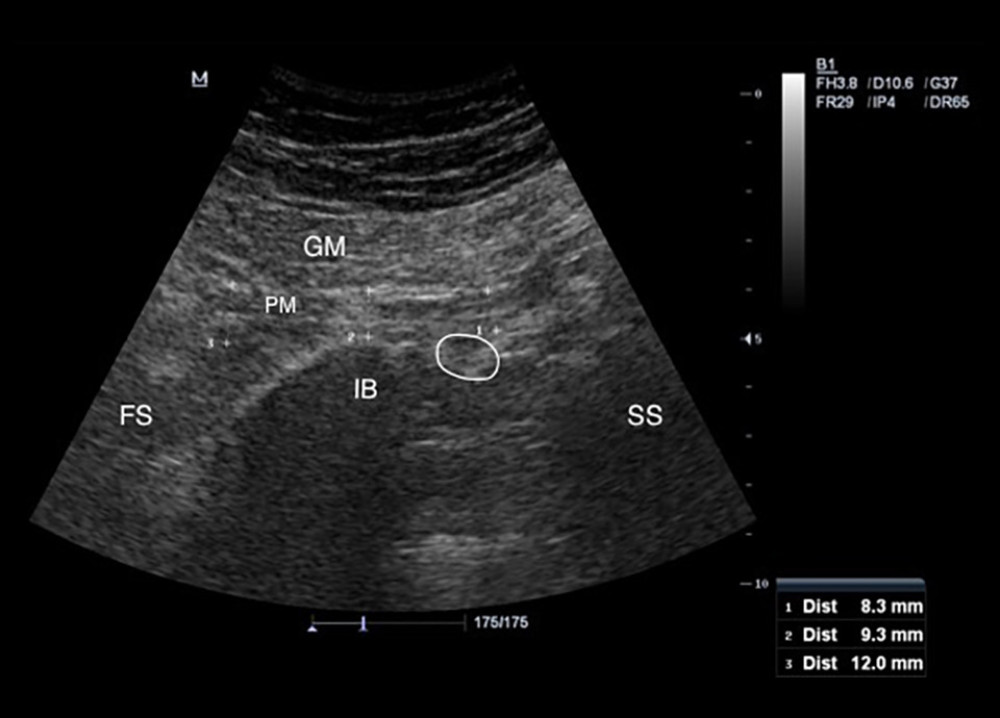 Figure 2. Measurement of the piriformis muscle thickness from 3 sites. Thickest parts of the piriformis muscle over the ilium, on the lateral side of the ilium near greater trochanter, and on the medial side of the ilium near the sacrum were measured. GM – gluteus maximus muscle; PM – piriformis muscle; IB – iliac bone; SS – sacral side; FS – femoral side; circle – sciatic nerve.
Figure 2. Measurement of the piriformis muscle thickness from 3 sites. Thickest parts of the piriformis muscle over the ilium, on the lateral side of the ilium near greater trochanter, and on the medial side of the ilium near the sacrum were measured. GM – gluteus maximus muscle; PM – piriformis muscle; IB – iliac bone; SS – sacral side; FS – femoral side; circle – sciatic nerve. 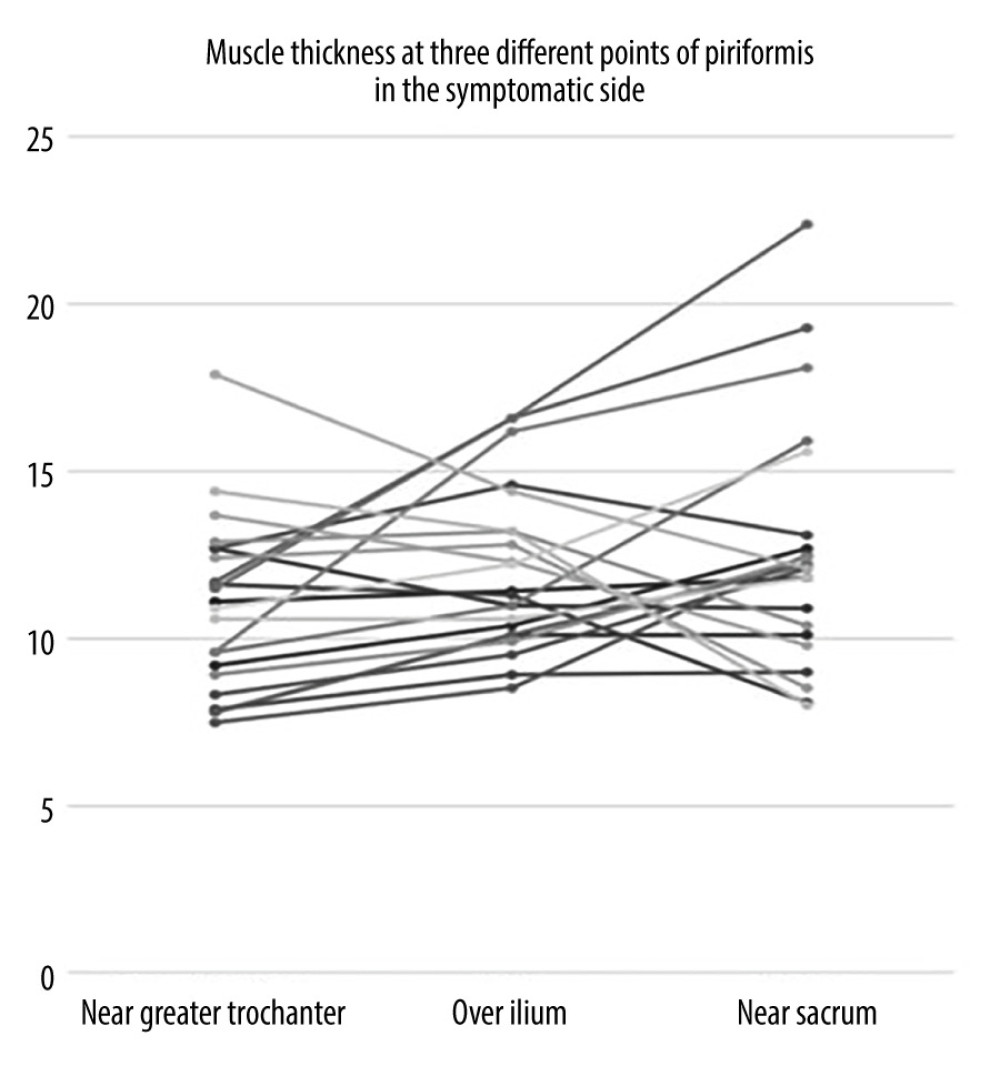 Figure 3. Individual values for muscle thickness at 3 different points of the piriformis in the symptomatic side of patients with piriformis syndrome.
Figure 3. Individual values for muscle thickness at 3 different points of the piriformis in the symptomatic side of patients with piriformis syndrome. 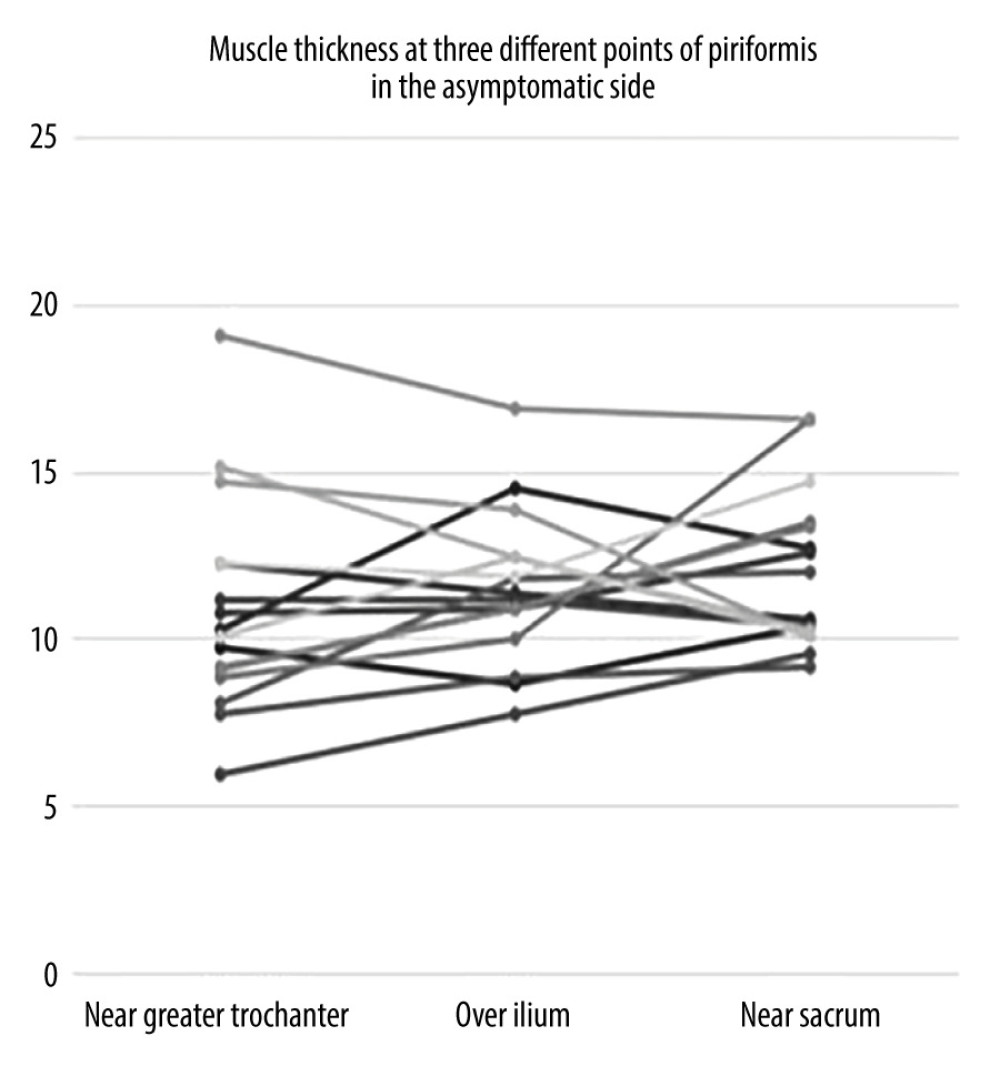 Figure 4. Individual values for muscle thickness at 3 different points of the piriformis in the asymptomatic side of patients with piriformis syndrome.
Figure 4. Individual values for muscle thickness at 3 different points of the piriformis in the asymptomatic side of patients with piriformis syndrome. Tables
Table 1. Individual measurement values of piriformis muscle thickness measurements from different measurement sites in dominant and non-dominant sides of patients with piriformis syndrome by each examiner.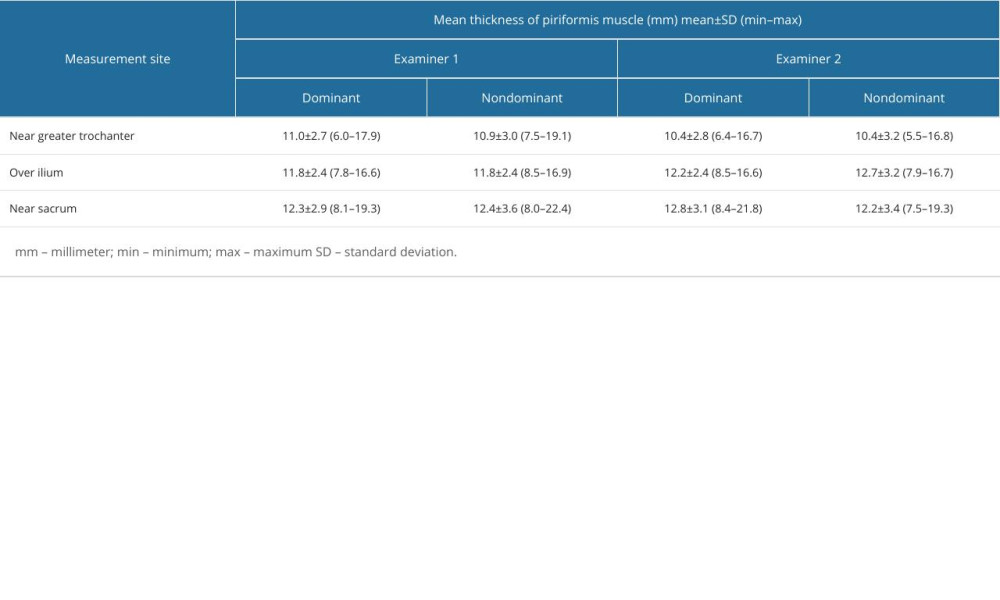 Table 2. Interrater reliability of muscle thickness measurements between 2 examiners.
Table 2. Interrater reliability of muscle thickness measurements between 2 examiners.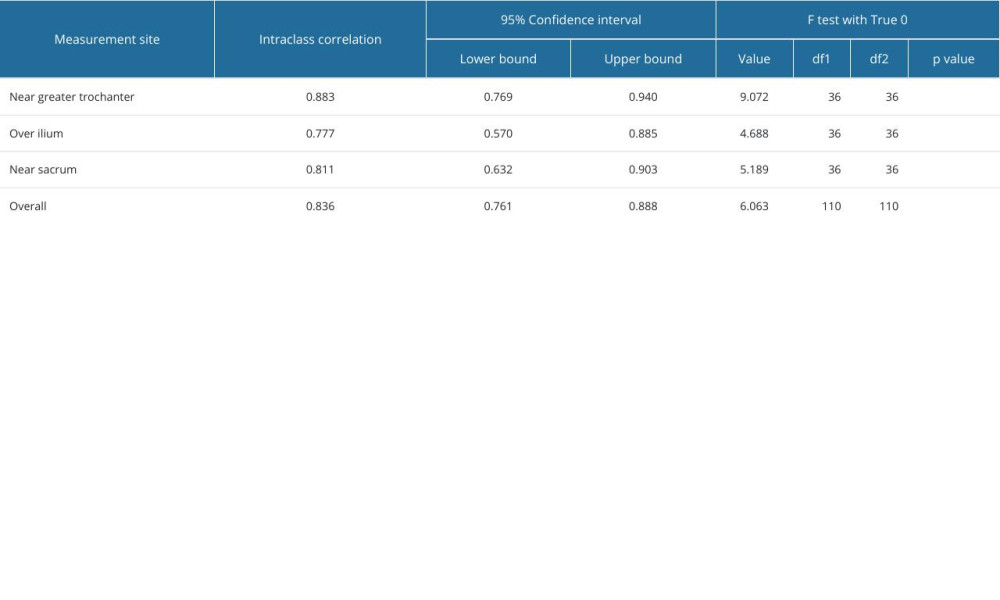 Table 3. Individual measurement values of piriformis muscle thickness measurements from different measurement sites in symptomatic and normal sides of patients with piriformis syndrome by each examiner.
Table 3. Individual measurement values of piriformis muscle thickness measurements from different measurement sites in symptomatic and normal sides of patients with piriformis syndrome by each examiner.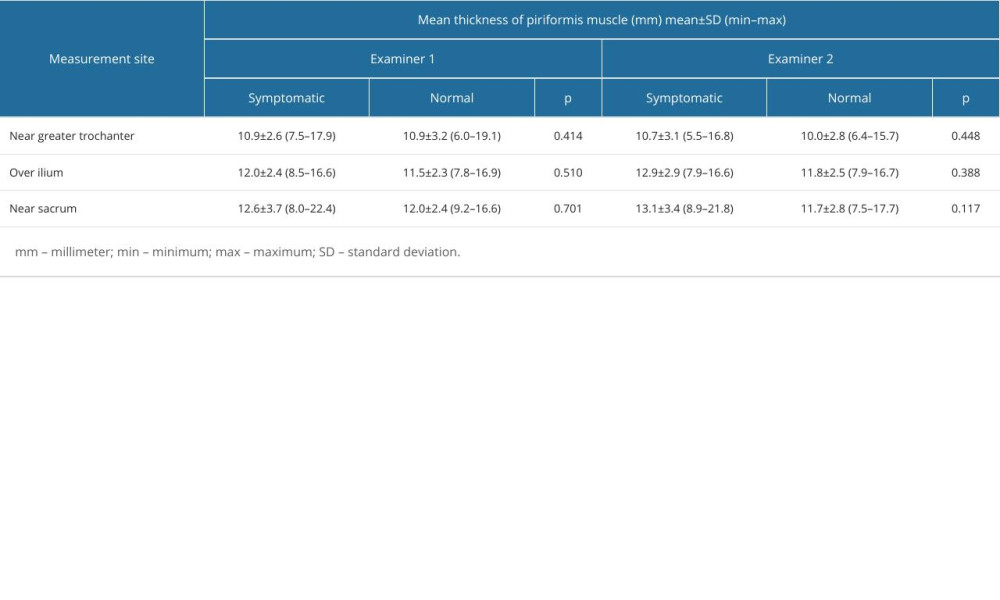
References
1. Chang C, Jeno SH, Varacallo M, Anatomy, bony pelvis and lower limb. Piriformis muscle [Updated 2023 Nov 13]: StatPearls [Internet], 2023, Treasure Island (FL), StatPearls Publishing Available from: https://www.ncbi.nlm.nih.gov/books/NBK519497/
2. Papadopoulos EC, Khan SN, Piriformis syndrome and low back pain: A new classification and review of the literature: Orthop Clin North Am, 2004; 35; 65-71
3. Hopayian K, Danielyan A, Four symptoms define the piriformis syndrome: An updated systematic review of its clinical features: Eur J Orthop Surg Traumatol, 2018; 28; 155164
4. Vas L, Pai R, Pawar KS, Pattnaik M, “Piriformis syndrome”: Is it only piriformis?: Pain Med, 2016; 17; 1775-79
5. Carro LP, Hernando MF, Cerezal L, Deep gluteal space problems: Piriformis syndrome, ischiofemoral impingement and sciatic nerve release: Muscles Ligaments Tendons J, 2016; 6(3); 384-96
6. Hicks BL, Lam JC, Varacallo M, Piriformis syndrome. [Updated 2023 Aug 4[: StatPearls [Internet], 2023, Treasure Island (FL), StatPearls Publishing Available from: https://www.ncbi.nlm.nih.gov/books/NBK448172/
7. Kirschner JS, Foye PM, Cole JL, Piriformis syndrome, diagnosis and treatment: Muscle Nerve, 2009; 40; 10-18
8. Rossi P, Cardinali P, Serrao M, Magnetic resonance imaging findings in piriformis syndrome: A case report: Arch Phys Med Rehabil, 2001; 82; 519-21
9. Vassalou EE, Katonis P, Karantanas AH, Piriformis muscle syndrome: A cross-sectional imaging study in 116 patients and evaluation of therapeutic outcome: Eur Radiol, 2018; 28; 447-58
10. Chang KV, Wu WT, Lew HL, Özçakar L, Ultrasound imaging and guided injection for the lateral and posterior hip: Am J Phys Med Rehabil, 2018; 97; 285-91
11. Zhang W, Luo F, Sun H, Ding H, Ultrasound appears to be a reliable technique for the diagnosis of piriformis syndrome: Muscle Nerve, 2019; 59; 411-16
12. Wu YY, Guo XY, Chen K, Feasibility and reliability of an ultrasound examination to diagnose piriformis syndrome: World Neurosurg, 2020; 134; e1085-e92
13. Siahaan YMT, Tiffani P, Tanasia A, Ultrasound-guided measurement of piriformis muscle thickness to diagnose piriformis syndrome: Front Neurol, 2021; 12; 721966
14. Othman IK, Raj NB, Siew Kuan C, Association of piriformis thickness, hip muscle strength, and low back pain patients with and without piriformis syndrome in Malaysia: Life (Basel), 2023; 13; 1208
15. Xu M, Meng B, Geng Z, A case-control study of 40 patients with piriformis muscle syndrome to evaluate diagnostic findings using two-dimensional ultrasound and shear wave elastography: Med Sci Monit, 2023; 29; e940214
16. Demirel A, Baykara M, Koca TT, Berk E, Ultrasound elastography findings in piriformis muscle syndrome: Indian J Radiol Imaging, 2018; 28; 412-18
17. Battaglia PJ, Mattox R, Haun DW, Dynamic ultrasonography of the deep external rotator musculature of the hip: A descriptive study: PM R, 2016; 8(7); 640-50
18. Arifin WN: Sample size calculator (web) [Internet], 2024 [cited 2 February 2024]. Available from: http://wnarifin.github.io
19. Koo TK, Li MY, A guideline of selecting and reporting intraclass correlation coefficients for reliability research: J Chiropr Med, 2016; 15; 155-63 [Erratum in: J Chiropr Med. 2017;16:346]
20. Broadhurst NA, Simmons DN, Bond MJ, Piriformis syndrome: Correlation of muscle morphology with symptoms and signs: Arch Phys Med Rehabil, 2004; 85; 2036-39
21. Grimaldi A, Richardson C, Stanton W, The association between degenerative hip joint pathology and size of the gluteus medius, gluteus minimus and piriformis muscles: Man Ther, 2009; 14; 605-10
22. Skorupska E, Keczmer P, Lochowski RM, Reliability of MR-based volumetric 3-D analysis of pelvic muscles among subjects with low back with leg pain and healthy volunteers: PLoS One, 2016; 11; e0159587
23. Eastlack J, Tenorio L, Wadhwa V, Sciatic neuromuscular variants on MR neurography: Frequency study and interobserver performance: Br J Radiol, 2017; 90; 20170116
24. Russell JM, Kransdorf MJ, Bancroft LW, Magnetic resonance imaging of the sacral plexus and piriformis muscles: Skeletal Radiol, 2008; 37; 709-13
25. Filler AG, Haynes J, Jordan SE, Sciatica of nondisc origin and piriformis syndrome: diagnosis by magnetic resonance neurography and interventional magnetic resonance imaging with outcome study of resulting treatment: J Neurosurg Spine, 2005; 2; 99-115
26. Haladaj R, Pingot M, Polguj M, Anthropometric study of the piriformis muscle and sciatic nerve: A morphological analysis in a Polish population: Med Sci Monit, 2015; 21; 3760-68
27. Park CH, Lee SH, Lee SC, Park HS, Piriformis muscle: Clinical anatomy with computed tomography in korean population: Korean J Pain, 2011; 24; 87-92
Figures
 Figure 1. Placement of the ultrasound transducer over the piriformis muscle. The patient is lying in prone position with lower extremity in neutral position. Thickness measurement of piriformis muscle is performed in the longitudinal view.
Figure 1. Placement of the ultrasound transducer over the piriformis muscle. The patient is lying in prone position with lower extremity in neutral position. Thickness measurement of piriformis muscle is performed in the longitudinal view. Figure 2. Measurement of the piriformis muscle thickness from 3 sites. Thickest parts of the piriformis muscle over the ilium, on the lateral side of the ilium near greater trochanter, and on the medial side of the ilium near the sacrum were measured. GM – gluteus maximus muscle; PM – piriformis muscle; IB – iliac bone; SS – sacral side; FS – femoral side; circle – sciatic nerve.
Figure 2. Measurement of the piriformis muscle thickness from 3 sites. Thickest parts of the piriformis muscle over the ilium, on the lateral side of the ilium near greater trochanter, and on the medial side of the ilium near the sacrum were measured. GM – gluteus maximus muscle; PM – piriformis muscle; IB – iliac bone; SS – sacral side; FS – femoral side; circle – sciatic nerve. Figure 3. Individual values for muscle thickness at 3 different points of the piriformis in the symptomatic side of patients with piriformis syndrome.
Figure 3. Individual values for muscle thickness at 3 different points of the piriformis in the symptomatic side of patients with piriformis syndrome. Figure 4. Individual values for muscle thickness at 3 different points of the piriformis in the asymptomatic side of patients with piriformis syndrome.
Figure 4. Individual values for muscle thickness at 3 different points of the piriformis in the asymptomatic side of patients with piriformis syndrome. Tables
 Table 1. Individual measurement values of piriformis muscle thickness measurements from different measurement sites in dominant and non-dominant sides of patients with piriformis syndrome by each examiner.
Table 1. Individual measurement values of piriformis muscle thickness measurements from different measurement sites in dominant and non-dominant sides of patients with piriformis syndrome by each examiner. Table 2. Interrater reliability of muscle thickness measurements between 2 examiners.
Table 2. Interrater reliability of muscle thickness measurements between 2 examiners. Table 3. Individual measurement values of piriformis muscle thickness measurements from different measurement sites in symptomatic and normal sides of patients with piriformis syndrome by each examiner.
Table 3. Individual measurement values of piriformis muscle thickness measurements from different measurement sites in symptomatic and normal sides of patients with piriformis syndrome by each examiner. Table 1. Individual measurement values of piriformis muscle thickness measurements from different measurement sites in dominant and non-dominant sides of patients with piriformis syndrome by each examiner.
Table 1. Individual measurement values of piriformis muscle thickness measurements from different measurement sites in dominant and non-dominant sides of patients with piriformis syndrome by each examiner. Table 2. Interrater reliability of muscle thickness measurements between 2 examiners.
Table 2. Interrater reliability of muscle thickness measurements between 2 examiners. Table 3. Individual measurement values of piriformis muscle thickness measurements from different measurement sites in symptomatic and normal sides of patients with piriformis syndrome by each examiner.
Table 3. Individual measurement values of piriformis muscle thickness measurements from different measurement sites in symptomatic and normal sides of patients with piriformis syndrome by each examiner. In Press
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
11 Mar 2024 : Clinical Research
Comparison of Effects of Sugammadex and Neostigmine on Postoperative Neuromuscular Blockade Recovery in Pat...Med Sci Monit In Press; DOI: 10.12659/MSM.942773
12 Mar 2024 : Clinical Research
Comparing Neuromuscular Blockade Measurement Between Upper Arm (TOF Cuff®) and Eyelid (TOF Scan®) Using Miv...Med Sci Monit In Press; DOI: 10.12659/MSM.943630
11 Mar 2024 : Clinical Research
Enhancement of Frozen-Thawed Human Sperm Quality with Zinc as a Cryoprotective AdditiveMed Sci Monit In Press; DOI: 10.12659/MSM.942946
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








