11 May 2024: Clinical Research
Differential DHA and EPA Levels in Women with Preterm and Term Births: A Tertiary Hospital Study in Indonesia
Muhammad Alamsyah AzizDOI: 10.12659/MSM.943895
Med Sci Monit 2024; 30:e943895
Abstract
BACKGROUND: Preterm birth is one of the main causes of neonatal death worldwide. One strategy focused on preventing preterm birth is the administration of long chain polyunsaturated fatty acids (LCPUFAs) during pregnancy. Omega-3 LCPUFAs, including eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), are essential in metabolic and physiological processes during embryonic and fetal development. This study aimed to compare DHA and EPA levels in 44 women with preterm births and 44 women with term births at a tertiary hospital in West Java Province, Indonesia, between November 2022 and March 2023.
MATERIAL AND METHODS: A total of 88 patients in this study consisted of 44 patients with term births (≥37 gestational weeks) and 44 patients with preterm births (<37 gestational weeks) at a tertiary hospital in West Java Province, Indonesia. This observational, cross-sectional study was conducted from November 2022 to March 2023. Using the enzyme-linked immunosorbent assay test, maternal DHA and EPA levels were investigated. IBM SPSS 24.0 was used to statistically measure outcomes.
RESULTS: Average maternal DHA and EPA levels in patients with preterm births were significantly lower than those in term births. Preterm labor risk was further increased by DHA levels of ≤5.70 µg/mL (OR=441.00, P=0.000) and EPA levels ≤3971.54 µg/mL (OR=441.00, P=0.000).
CONCLUSIONS: Since the average maternal DHA and EPA levels were significantly lower in patients with preterm births, adequate intake of omega-3 LCPUFA in early pregnancy and consistency with existing nutritional guidelines was associated with a lower risk of preterm delivery for pregnant women.
Keywords: 6,7-dihydroxy-1,2,3,4,8,12b-hexahydroanthr(10,4a,4-cd)azepine, Eicosapentaenoate-Lipoate, Premature Birth
Introduction
Preterm neonates are defined as fetuses born before 37 weeks of gestation, while term births are fetuses born after 37 gestational weeks. Preterm birth is also the highest cause of death in newborn babies, accounting for more than 85% of all cases of perinatal complications and deaths [1–5]. With 675 700 cases and a ratio of 15.5 per 100 live births, Indonesia is one of the 10 nations with the highest number of preterm births worldwide [2,3]. With respect to local data, a case study conducted in a West Java tertiary hospital demonstrated that 70% of all infant deaths that occurred at a tertiary hospital in West Java Province, Indonesia, were cases of preterm birth [6].
Preterm birth is significantly influenced by a number of risk factors, including low maternal weight, obesity, anemia, malnutrition, inability to obtain dietary supplements, and low income. Progesterone therapy and cervical cerclage are 2 of the many methods that have been used to lower the risk of premature birth. Nevertheless, there are restrictions on this method’s availability, efficiency, and safety [1–6]. Giving pregnant women long-chain polyunsaturated fatty acids (LCPUFAs) is one method of preventing preterm birth that is readily available, reasonably priced, and has few adverse effects. During embryonic and fetal development, omega-3 LCPUFAs, such as docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), are crucial for metabolic and physiological activities [7,8].
DHA and EPA are two examples of omega-3 [(n-3)] long-chain polyunsaturated fats (PUFAs) that have several health advantages, especially for fetal development [9]. For pregnancy, DHA and EPA can prevent preterm birth through the following mechanisms. DHA has cellular effects that can change the onset or timing of inflammation and promote the activation of SIRT1 expression, which has a direct impact on endothelial relaxation or changes membrane fluidity and cell signaling [10,11]. DHA released from cell membrane phospholipids functions as a precursor of docosanoids (22 carbon mediators, such as resolving, protectin, and maresin), which are anti-inflammatory, overcome inflammation, and protect against inflammation. EPA increases the duration of pregnancy because it is competitive with arachidonic acid, a source of prostaglandins E2 and F2α, which is needed and increases during labor [10–14]. In addition to preventing preterm birth, omega-3 requirements are higher than normal to support fetal growth, especially of the brain and eyes. Pregnancy-related deficiency in omega-3 fatty acids has been linked to behavioral and visual abnormalities in animals, which cannot be corrected by postnatal supplementation [12,13].
Plasma EPA and DHA concentrations below 2.0% to 2.5% will significantly increase the risk of early preterm birth. EPA and DHA levels are usually measured as the omega-3 index, which is the level of EPA and DHA in erythrocytes. The target omega-3 index level for pregnant and breastfeeding women is 8% to 11% [8–10]. Although higher intakes of DHA and EPA have been recommended worldwide, dietary reference intakes have not been established. The range of EPA-DHA supplementation doses generally recommended for pregnant women is relatively wide, from 200 mg/day to 1000 mg/day. The Institute of Medicine recommends EPA and DHA intake of at least 10% of total omega-3 fatty acid intake (approximately 160 mg per day). Meanwhile, the Food and Agriculture Organization recommends a DHA intake of 200 mg/day during pregnancy [9,10].
Omega-3 fatty acids can be obtained from consuming fish, such as tuna, salmon, and sardines, or can also be obtained from fish oil supplements [15]. However, currently fish consumption in various countries is still low, and women of childbearing age tend to be reluctant to increase fish consumption, due to the perception that the mercury content and other pollutants in fish can have a negative impact on the fetus [1]. Fish consumption in Indonesia tends to be low compared with that of other countries in Southeast Asia, even though fish production in Indonesia is higher than that of other countries. Based on a report from the Ministry of Maritime Affairs and Fisheries Republic of Indonesia in 2022, people’s consumption of fish was around 56.48 kg per capita per year, although this figure had increased from the previous 55.16 kg per capita per year in 2021 [16].
Based on a study conducted by Saccone et al, pregnant women who received omega-3 supplementation containing DHA and EPA had a longer gestational age and their babies had a greater birth weight than the control group [17]. Apart from that, other studies also show that consuming DHA and EPA supplementation could increase fetal weight [17,18]. Cohort studies in Norway and Australia also concluded that children had higher mental processing and cognition scores when born to mothers who were fed foods high in DHA and EPA levels during their pregnancy and breastfeeding [19,20]. Several studies examining EPA and DHA intake during pregnancy, and their correlation with longer gestational age, indicate that EPA and DHA supplementation during pregnancy delays the onset of labor until the optimal gestational age [21–23].
Therefore, preterm birth and other health problems in mother and baby are correlated with blood levels of EPA and DHA, and the numbers are predicted to be reduced by increasing EPA and DHA intake. However, very high intake or levels of EPA and DHA can also cause health problems, such as bleeding, prolonged pregnancy, or even preterm birth [8–15]. The variations in EPA and DHA levels between pregnant women who give birth at term and those who give birth before term have not yet been explained by previous studies. Thus, this study aimed to compare DHA and EPA levels in 44 women with preterm births and 44 women with term births at a tertiary hospital in West Java Province, Indonesia, between November 2022 and March 2023.
Material and Methods
ETHICAL STATEMENT, STUDY DESIGN, AND RESEARCH PARTICIPANTS:
The approval and recommendations of the tertiary hospital’s ethics committee review board (reference number LB.02.01/X.6.5/386/2022) were obtained before any procedures were conducted, in compliance with applicable norms and regulations. The study was conducted as per the Declaration of Helsinki. Each patient provided informed consent. Details regarding the trial were explained to patients both written or orally.
This was a descriptive analytical study conducted using a cross-sectional method on women who gave birth to their babies at the age of 24+0 to 41+6 weeks and delivered at tertiary hospitals in West Java Province, Indonesia, from the end of October 2022 to March 2023. Participants were considered included if they met the inclusion and exclusion criteria.
INCLUSION AND EXCLUSION CRITERIA:
This study included women aged ≥18 years who gave birth through vaginal birth to their babies at 24+0 to 41+6 gestational weeks and delivered at the tertiary hospital in West Java Province, Indonesia, without fetal abnormalities. Through the completion of an informed consent form, they granted permission to take part in the study. Exclusion criteria consisted of patients with comorbidities, and refusal to participate.
DATA COLLECTION METHODS:
Preterm and term births comprised the 2 groups of the total 86 women who fulfilled the study criteria and gave vaginal birth. Consecutive samplings were used to obtain each sample. The tertiary hospital in West Java Province, Indonesia, used primary data from patients who arrived and gave birth at both preterm and term gestational weeks. These patients were admitted through the Emergency Room or the Obstetrics and Gynecology Polyclinic. Blood samples were obtained from women who had given birth, and the hospital’s Clinical Pathology Laboratory assessed the EPA and DHA levels. Patients who fulfilled the study requirements had their antenatal care (ANC) history and supplementation during pregnancy taken. Every patient who gave vaginal birth in preterm or term manner had blood drawn to assess DHA and EPA levels in the blood.
Maternal DHA and EPA levels were measured in the laboratory using the enzyme-linked immunosorbent assay (ELISA) method. A 96-well plate was pre-coated with an antibody. The wells were filled with standards, test samples, and biotin-conjugated reagent before being incubated. The biotin-labeled DHA and the unlabeled DHA on the pre-coated antibody underwent a competitive inhibition reaction. After adding the horseradish peroxidase-conjugated reagent, the plate was incubated as a whole. At each step, unbound conjugates were eliminated with washing buffer. The horseradish peroxidase enzymatic reaction was quantified using 3,3′,5,5′-tetramethylbenzidine (TMB) substrate. Only wells with enough DHA yielded a blue product after the TMB substrate was added; this product turned yellow when the acidic stop solution was added. In a microplate reader, the optical density was measured spectrophotometrically at 450 nm to determine the DHA concentration. The material was centrifuged for 15 min at 1000×g and at 2°C to 8°C after being allowed to settle for a maximum of 30 min at room temperature. The supernatant was collected to measure the levels of EPA and DHA. If the inspection had not been completed right away, the supernatant was kept in storage at either −20°C for 7 days or −81°C for 1 month.
STATISTICAL ANALYSIS:
The results of the examination were recorded and analyzed. The outcomes of the examination were noted and analyzed. The Mann-Whitney U or independent
Results
PATIENT CHARACTERISTICS:
The following patient data, considered as risk factors for preterm labor, were retrieved: maternal age, residential area, parity, smoking status, and number of ANC variables (Table 1). Number of parity was considered as “with risks” if >3. The statistical tests on all variables were not statistically significant (P>0.05). No differences in the proportion of maternal age, residential area, parity, smoking status, and number of ANC variables were found.
COMPARISON OF MEAN DHA AND EPA LEVELS IN PRETERM VS TERM BIRTH:
The mean EPA levels in preterm births and term births were 3137.77±650.89 μg/mL and 5526.15±4771.87 μg/mL, respectively. Compared with term births, preterm births had a lower mean EPA level. This difference was statistically significant, at P=0.000, according to the Mann-Whitney U test results. In preterm births, the mean DHA level was 3.17±1.93 μg/mL, while in term births, it was 11.63±11.53 μg/mL. Preterm births had a lower mean DHA level than did term births. This difference was statistically significant, at P=0.000, according to the Mann-Whitney U test results (Table 2). A cut-off point was determined using ROC analysis, since there was no cut-off point for EPA and DHA levels obtained in our study center.
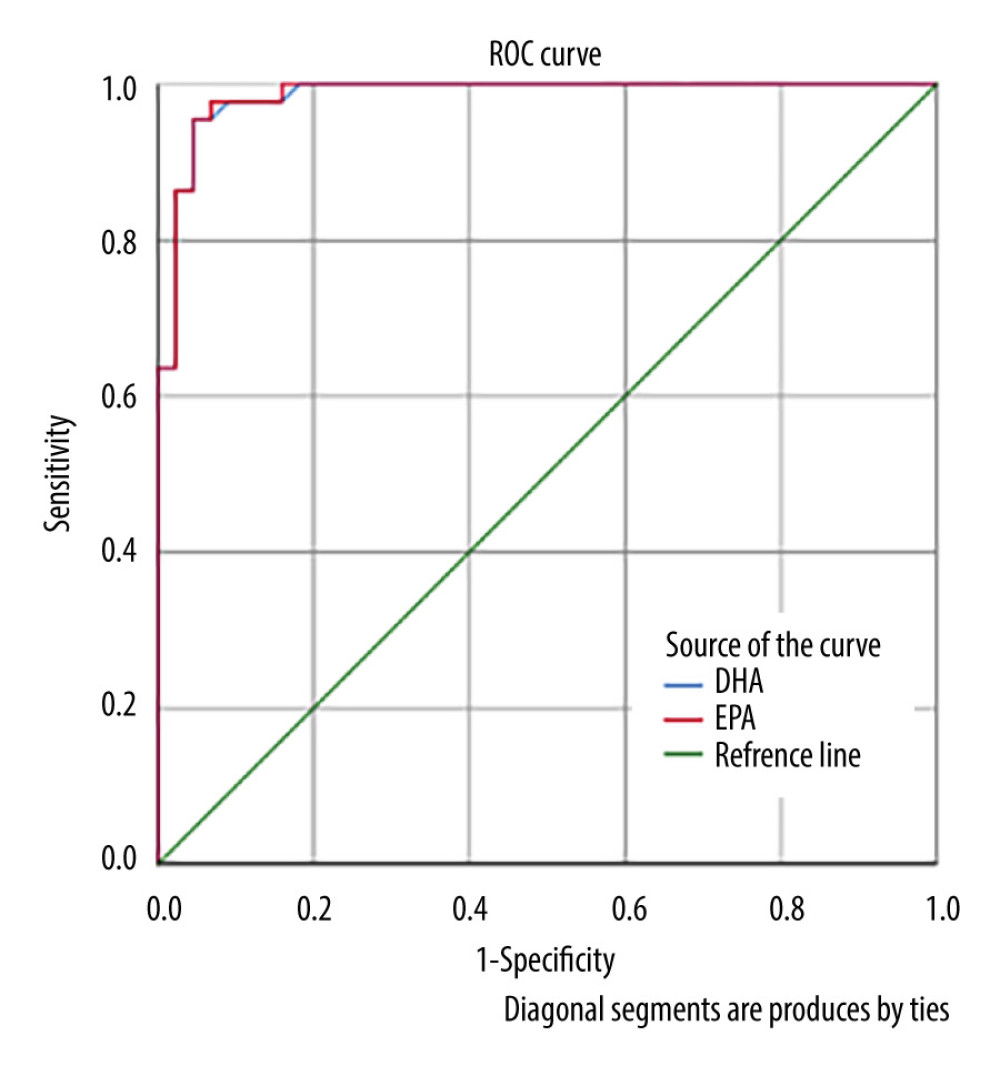
As shown in Figure 1, the ROC curve for EPA and DHA was above the 50% line, so this curve had a good probability value. The area under the ROC curve (AUC) results for DHA was 98.5% (P=0.000; Figure 1). The interpretation of the AUC value of 98.5% was considered very good, indicating that the diagnostic value of DHA for predicting a term pregnancy was 98.5%. The EPA AUC result was 98.6% (P=0.000), which was also considered very good, indicating that the diagnostic value of EPA for predicting a term pregnancy was 98.6% (Table 3).
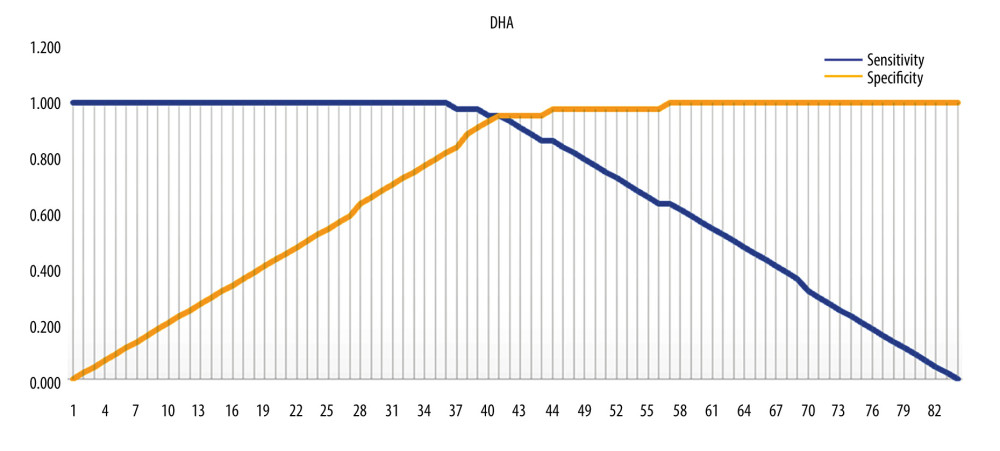
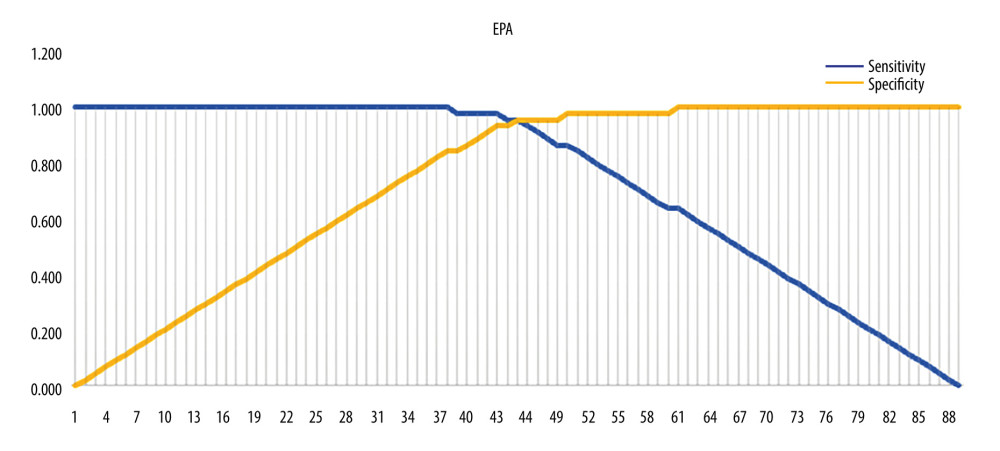
The optimal cut-off point of DHA was 41 (Figure 2), with a value of 5.70, sensitivity of 95.5%, and specificity of 95.5%, indicating that pregnant women with a DHA score >5.70 μg/mL have a high probability of giving birth at term. For EPA, the optimal cut-off point was 45, with a value of 3971.54 μg/mL, sensitivity of 95.5%, and specificity of 95.5%, indicating that pregnant women with an EPA score >3971.54 have a high probability of giving birth at term. For EPA, the optimal cut-off point was 45 (Figure 3).
COMPARISON OF PRETERM BIRTH RISKS BASED ON MEAN DHA AND EPA LEVELS:
Pregnant women with DHA levels ≤5.70 μg/mL had an odds ratio (OR) of 441 times that of pregnant women with DHA levels >5.70 μg/mL for preterm birth. Thus, pregnant women with DHA levels ≤5.70 μg/mL had a 441 times higher risk of preterm birth than pregnant women with DHA levels >5.70 μg/mL. Pregnant women with EPA levels ≤3971.54 μg/mL had an OR of 441 times that of pregnant women with EPA levels >3971.54 μg/mL for preterm birth. Pregnant women with EPA levels ≤3971.54 μg/mL had a 441 times higher risk of preterm birth than pregnant women with EPA levels >3971.54 μg/mL. DHA levels less than 5.70 μg/mL and EPA levels less than 3971.54 μg/mL increased the risk of preterm birth (Table 4).
Discussion
No differences in proportions of maternal age, residential area, parity, smoking status, and number of ANC variables were found in this study. In line to research conducted by Jung et al, higher maternal age, education level, occupation, passive smoking, iron intake, and parity also showed no significant differences between full-term births and preterm births. Specifically, women who were exposed to passive smoking (odds ratio [OR]=2.83, confidence interval [CI]=1.14–7.04) and had folic acid intake during pregnancy (OR=2.67, CI=1.11–6.43) were in significantly increased risk of preterm birth, after adjusting for all variables [24]. The relationship between residential area and preterm birth is assumed to be due to environmental factors, including socio-economic conditions and environmental conditions, such as pollution levels and water quality. Access to health services and the presence of health workers who can help mothers must also be considered [25–27]. A study conducted by Malwela et al [28], in rural areas in South Africa, shows that the cause of the increasing incidence of preterm births is the availability of equipment, which is less than 40%, making preterm births unpreventable. This is also related to research by Pervin et al, in which pregnant women rarely had ANC [26]. In our research, the results of the chi-square test showed that there was no difference in results between region of origin and birth with a
In the present study, mothers with preterm births had lower average EPA levels than those with term births. These results provide an assessment of the contribution of omega-3 LCPUFAs to preterm birth, concerning dosage, timing of supplementation, omega-3, and examination of the relative DHA and EPA balance. These findings are similar to those of previous studies [8–17]. Moreover, mothers with omega-3 supplementation containing DHA and EPA have a longer gestational age and their babies have a greater birth weight compared with control groups [8–17]. Fatty acids are thought to play a role in the mechanism of preterm birth [24,31]. While the 2-series prostaglandins E2 and F2α (derived from arachidonic acid) are known to be involved in uterine contractions and cervical ripening, EPA, a homologous precursor of the 3-series prostaglandins, is much less effective. Furthermore, EPA and DHA both have the ability to relax the myometrium, delaying the early onset of labor, and to prevent the trophoblastic inflammatory pathway from being activated, which in turn reduces inflammation linked to some premature deliveries [24].
Mothers with preterm births have lower average DHA levels than do mothers with term births, on average, and results of the present study showed a statistically significant difference. In recent years, increasing attention has focused on the effects of maternal dietary intake of omega-3 LCPUFAs on perinatal outcomes, resulting in more than 70 completed randomized controlled trials. Collectively, the trials summarized in a recently published 2018 Cochrane review demonstrated improvements in several outcomes, including longer gestational duration, higher birth weight, and reduced risk of preterm birth and early preterm birth [3]. The average DHA level in preterm births was 3.17±1.93 μg/mL, and in term births was 11.63±11.53 μg/mL. Zhao et al [32] found that low circulating DHA levels can lead to impaired fetal insulin sensitivity, which may be involved in susceptibility to type 2 diabetes. It is also known that DHA and other fatty acids are associated with fetal insulin sensitivity and beta cell function. DHA supplementation in early pregnancy can prevent placental ischemia and internal placentation disorders (preeclampsia, fetal growth restriction) [32]. However, in the present study, preterm labor was more likely in cases in which DHA levels were ≤5.70 μg/mL and EPA levels were ≤3971.54 μg/mL.
Parturition is characterized by increased production of inflammatory mediators and the entry of leukocytes and macrophages into the uterine cavity. Fetal membranes are critical in contributing to the production of inflammatory cytokines, which are chemotactic against inflammatory cells. DHA and EPA have been mentioned in the literature as having anti-inflammatory effects on the fetal membranes, thereby reducing the production of cytokines associated with preterm labor [33,34].
Pregnancy has responded differently to omega-3 PUFA supplementation in clinical trials. A randomized trial study from Denmark [22] found that taking fish oil supplements benefited pregnancy and decreased the chance of preterm birth before 34 weeks of gestation (RR=0.69, 95% CI=0.49–0.99). Another Scandinavian trial [34] found no difference in gestational length or birth weight in pregnant women given fish oil supplements. Babies with the highest quartile of DHA present in cord blood in this trial had a gestation period that was 9.3 days longer than that of infants in the lowest quartile of DHA present.
The reduction of matrix metalloproteinase 9 activity in amnion treated with EPA and DHA was indicative of the presence of omega-3 polyunsaturated fatty acids. It is believed that matrix metalloproteinase 9 activity, which dramatically rises in the amniotic membrane during delivery, plays a role in membrane rupture [31,32]. The fetus’s only supply of this fatty acid comes from the mother’s body’s storage of EPA and DHA. The growing intrauterine fetus actively receives both EPA and DHA through the placenta [25–28]. Therefore, women with adequate omega-3 intake early in pregnancy should maintain such intake. Women who are low in omega-3 fatty acids will benefit the most from omega-3 LCPUFA supplements to reduce the risk of preterm birth [24].
The present study has several limitations, including the use of cross-sectional methods, lack of any intervention, and lack of randomized controlled and double-blind methods; therefore, it relied only on medical record data, which can cause bias. Also, the number of samples was limited, and we used immunochromatography ELISA methods. Nevertheless, further study is required to fully understand the mechanism of action in lengthening gestation before this is taken into consideration for public health implementation.
Conclusions
Compared with mothers with term births, mothers with preterm births had lower mean DHA and EPA levels. Additionally, the risk of preterm labor was increased by DHA levels ≤5.70 μg/mL and EPA levels ≤3971.54. The results of this study showed that adequate intake of omega-3 LCPUFA in early pregnancy reduced the risk of preterm birth. This study can serve as a foundation for more in-depth investigations that could help with the creation of international recommendations.
References
1. Cunningham FG, Leveno KJ, Bloom SL: Williams obstetrics, 2021, McGraw Hill Brasil Available from: https://www.mhprofessional.com/williams-obstetrics-26e-9781260462739-usa
2. World Health Organization: WHO fact sheet: Preterm birth, 2017, WHO Available from:https://www.who.int/en/news-room/fact-sheets/detail/preterm-birth
3. Middleton P, Gomersall JC, Gould JF, Omega-3 fatty acid addition during pregnancy: Cochrane Database Syst Rev, 2018; 11(11); CD003402
4. Carvajal JA, Docosahexaenoic acid supplementation early in pregnancy may prevent deep placentation disorders: Biomed Res Int, 2014; 2014; 526895
5. Agency of Health Research and Development: Report of Indonesian Basic Health Research, 2018, Ministry of Health Republic of Indonesia Available from: https://repository.badankebijakan.kemkes.go.id/id/eprint/3514/
6. Aziz MA: Preterm birth prevention strategies: vitamin D supplementation, challenges and hopes for the future, 2019, Department of Obstetrics and Gynecology Faculty of Medicine University of Padjadjaran;Bandung Sagung Seto
7. Alisjahbana A, Peeters R, Meheus A, Perinatal mortality and morbidity in rural West-Java, Indonesia. Part I: Vital statistics based on cross-sectional surveys: Paediatr Indones, 1990; 30(1–2); 1-11
8. Best KP, Gibson RA, Makrides M, ISSFAL statement number 7 – Omega-3 fatty acids during pregnancy to reduce preterm birth: Prostaglandins Leukot Essent Fatty Acids, 2022; 186; 102495
9. Swanson D, Block R, Mousa SA, Omega-3 fatty acids EPA and DHA: Health benefits throughout life: Adv Nutr, 2012; 3(1); 1-7
10. Carlson SE, Gajewski BJ, Valentine CJ, Assessment of DHA on reducing early preterm birth: The ADORE randomized controlled trial protocol: BMC Pregnancy Childbirth, 2017; 17(1); 62
11. Nordgren TM, Lyden E, Anderson-Berry A, Hanson CJN, Omega-3 fatty acid intake of pregnant women and women of childbearing age in the United States: Potential for deficiency?: Nutrients, 2017; 9(3); 197
12. Saccone G, Berghella V, Omega-3 supplementation to prevent recurrent preterm birth: A systematic review and metaanalysis of randomized controlled trials: Am J Obstet Gynecol, 2015; 213(2); 135-40
13. Coletta JM, Bell SJ, Roman AS, Omega-3 fatty acids and pregnancy: Rev Obstet Gynecol, 2010; 3(4); 163-71
14. von Schacky C, Omega-3 fatty acids in pregnancy – the case for a target Omega-3 index: Nutrients, 2020; 12(4); 898
15. Sun L, Li Y, Xie W, Xue X, Association between omega-3 fatty acid supplementation and lower risk of preterm delivery: A systematic review and meta-analysis: J Matern Fetal Neonatal Med, 2022; 35(12); 2294-303
16. Oktavilia S, Prayogi R, Abdulah R, Indonesian fish consumption: an analysis of dynamic panel regression model, IOP Publishing Available at:https://iopscience.iop.org/article/10.1088/1755-1315/246/1/0120051
17. Saccone G, Berghella V, Omega-3 supplementation to prevent recurrent preterm birth: A systematic review and metaanalysis of randomized controlled trials: Am J Obstet Gynecol, 2015; 213(2); 135-40
18. Kar S, Wong M, Rogozinska E, Thangaratinam S, Effects of omega-3 fatty acids in prevention of early preterm delivery: A systematic review and meta-analysis of randomized studies: Eur J Obstet Gynecol Reprod Biol, 2016; 198; 40-46
19. Grimm KA, Foltz JL, Blanck HM, Scanlon KS, Household income disparities in fruit and vegetable consumption by state and territory: Results of the 2009 Behavioral Risk Factor Surveillance System: J Acad Nutr Diet, 2012; 112(12); 2014-21
20. Judge M, Harel O, Lammi-Keefe C, Maternal consumption of a docosahexaenoic acid-containing functional food during pregnancy: Benefit for infant performance on problem-solving but not on recognition memory tasks at age 9 mo: Am J Clin Nutr, 2007; 85(6); 1572-77
21. Makrides M, Gibson R, McPhee A, Effect of DHA supplementation during pregnancy on maternal depression and neurodevelopment of young children: A randomized controlled trial: JAMA, 2010; 304(15); 1675-83
22. Olsen S, Osterdal M, Salvig J, Duration of pregnancy in relation to fish oil supplementation and habitual fish intake: A randomised clinical trial with fish oil: Eur J Clin Nutr, 2007; 61(8); 976-85
23. Carlson S, Colombo J, Gajewski B, DHA supplementation and pregnancy outcomes: Am J Clin Nutr, 2013; 97(4); 808-15
24. Jung S, Kwon S, Kwon M, Docosahexaenoic acid improves vascular function via up-regulation of SIRT1 expression in endothelial cells: Biochem Biophys Res Commun, 2013; 437(1); 114-19
25. Esposito G, Mauri P, Cipriani S, The role of maternal age on the risk of preterm birth among singletons and multiples: A retrospective cohort study in Lombardy, Norther Italy: BMC Pregnancy Childbirth, 2022; 22(1); 1-11
26. Pervin J, Rahman S, Rahman M, Association between antenatal care visit and preterm birth: A cohort study in rural Bangladesh: BMJ Open, 2020; 10(7); e036699
27. Li L, Ma J, Cheng Y, Feng L, Urban–rural disparity in the relationship between ambient air pollution and preterm birth: Int J Health Geogr, 2020; 19(1); 1-15
28. Malwela T, Maputle M, The preterm birth rate in a resource-stricken rural area of the Limpopo Province, South Africa: Nurs Res Rev, 2022; 12; 67-75
29. Beeckman K, Louckx F, Downe S, Putman K, The relationship between antenatal care and preterm birth: The importance of content of care: Eur J Public Health, 2013; 23(3); 366-71
30. Carlson SE, Gajewski BJ, Valentine CJ, Higher dose docosahexaenoic acid supplementation during pregnancy and early preterm birth: A randomised, double-blind, adaptive-design superiority trial: EClinicalMedicine, 2021; 36; 100905
31. Valentine CJ, Khan AQ, Brown AR, Higher-dose DHA supplementation modulates immune responses in pregnancy and is associated with decreased preterm birth: Nutrients, 2021; 13(12); 4248
32. Zhao JP, Levy E, Fraser WD, Circulating docosahexaenoic acid levels are associated with fetal insulin sensitivity: PLoS One, 2014; 9(1); e85054
33. Kim P, Zhong M, Kim YS, Long chain polyunsaturated fatty acids alter oxytocin signaling and receptor density in cultured pregnant human myometrial smooth muscle cells: PLoS One, 2012; 7(7); e41708
34. Frew L, Sugiarto N, Rajagopal S, The effect of omega-3 polyunsaturated fatty acids on the inflammatory response of the amnion: Prostaglandins Leukot Essent Fat Acids, 2013; 89(4); 221-25
Figures
Tables
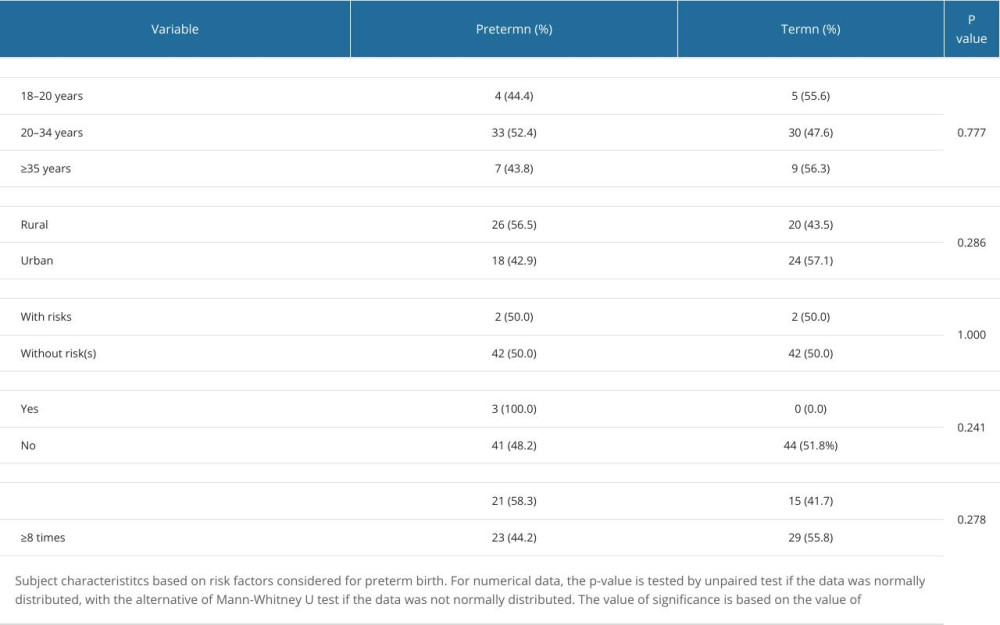 Table 1. Patient characteristics.
Table 1. Patient characteristics.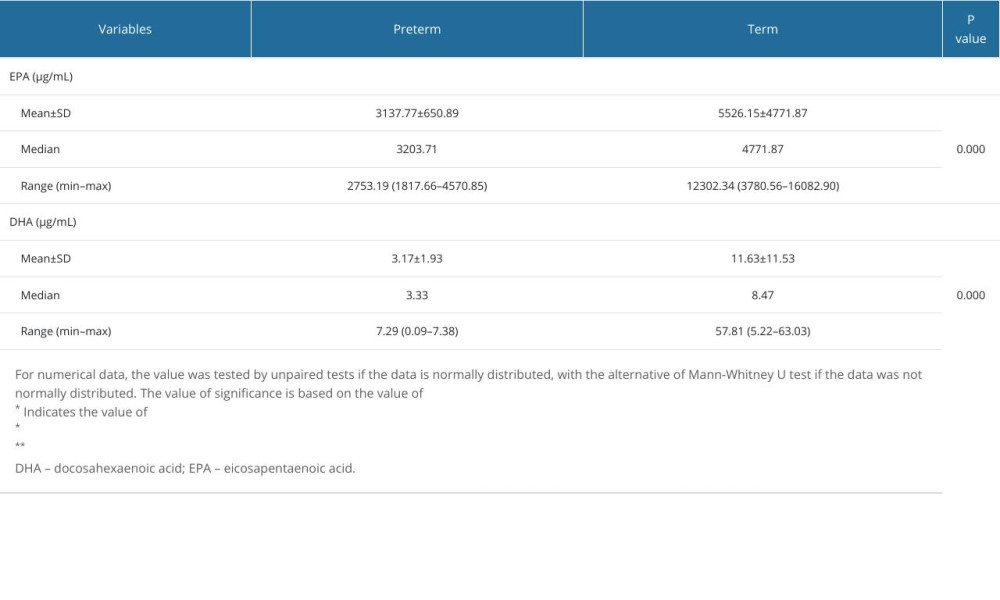 Table 2. Comparison in mean levels of DHA and EPA in preterm and term births.
Table 2. Comparison in mean levels of DHA and EPA in preterm and term births.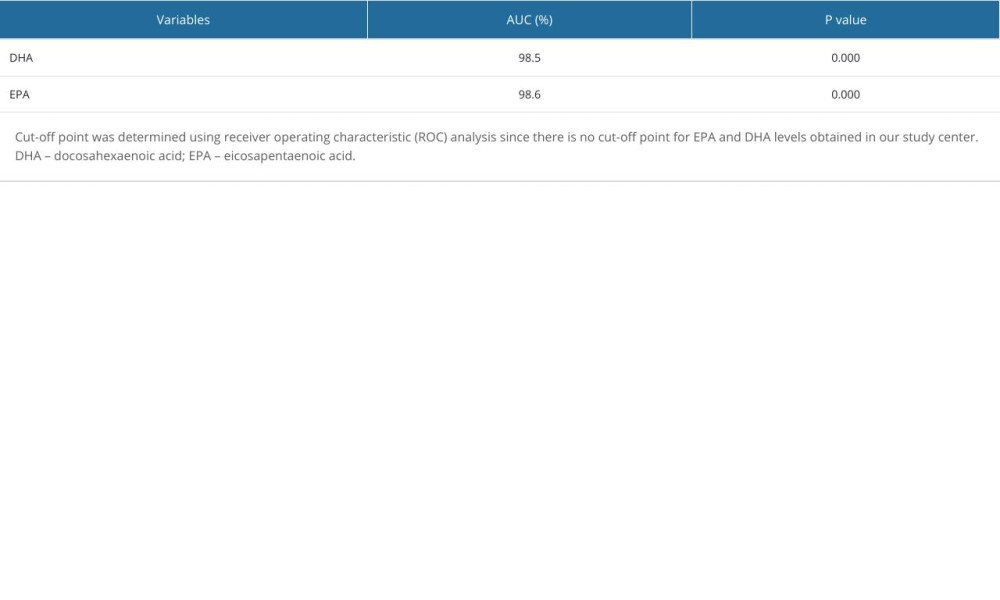 Table 3. Area under the ROC curve (AUC).
Table 3. Area under the ROC curve (AUC).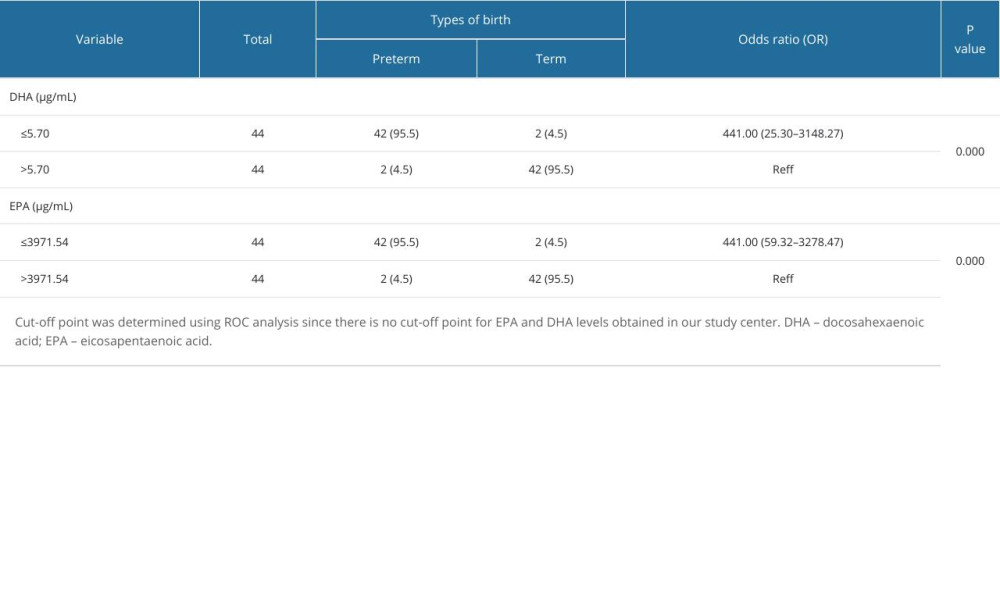 Table 4. Comparison of the risk of preterm and term birth based on DHA and EPA levels.
Table 4. Comparison of the risk of preterm and term birth based on DHA and EPA levels. Table 1. Patient characteristics.
Table 1. Patient characteristics. Table 2. Comparison in mean levels of DHA and EPA in preterm and term births.
Table 2. Comparison in mean levels of DHA and EPA in preterm and term births. Table 3. Area under the ROC curve (AUC).
Table 3. Area under the ROC curve (AUC). Table 4. Comparison of the risk of preterm and term birth based on DHA and EPA levels.
Table 4. Comparison of the risk of preterm and term birth based on DHA and EPA levels. In Press
16 Mar 2024 : Clinical Research
Diagnostic Efficiency of ACR-TIRADS Score for Differentiating Benign and Malignant Thyroid Nodules of Vario...Med Sci Monit In Press; DOI: 10.12659/MSM.943228
08 May 2024 : Clinical Research
Effect of Individualized PEEP Guided by Driving Pressure on Diaphragm Function in Patients Undergoing Lapar...Med Sci Monit In Press; DOI: 10.12659/MSM.944022
21 Mar 2024 : Clinical Research
Impact of Serum Vitamin D, B6, and B12 and Cognitive Functions on Quality of Life in Peri- and Postmenopaus...Med Sci Monit In Press; DOI: 10.12659/MSM.943249
17 Mar 2024 : Clinical Research
Protective Role of TRPC3 Gene Polymorphism (rs10518289) in Obstructive Sleep Apnea Hypopnea Syndrome Among ...Med Sci Monit In Press; DOI: 10.12659/MSM.942667
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952